A spatiotemporally resolved infection risk model for airborne transmission of COVID-19 variants in indoor spaces
- PMID: 34954184
- PMCID: PMC8695516
- DOI: 10.1016/j.scitotenv.2021.152592
A spatiotemporally resolved infection risk model for airborne transmission of COVID-19 variants in indoor spaces
Abstract
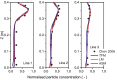
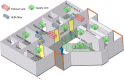
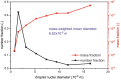
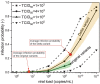
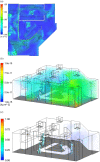
The classic Wells-Riley model is widely used for estimation of the transmission risk of airborne pathogens in indoor spaces. However, the predictive capability of this zero-dimensional model is limited as it does not resolve the highly heterogeneous spatiotemporal distribution of airborne pathogens, and the infection risk is poorly quantified for many pathogens. In this study we address these shortcomings by developing a novel spatiotemporally resolved Wells-Riley model for prediction of the transmission risk of different COVID-19 variants in indoor environments. This modelling framework properly accounts for airborne infection risk by incorporating the latest clinical data regarding viral shedding by COVID-19 patients and SARS-CoV-2 infecting human cells. The spatiotemporal distribution of airborne pathogens is determined via computational fluid dynamics (CFD) simulations of airflow and aerosol transport, leading to an integrated model of infection risk associated with the exposure to SARS-CoV-2, which can produce quantitative 3D infection risk map for a specific SARS-CoV-2 variant in a given indoor space. Application of this model to airborne COVID-19 transmission within a hospital ward demonstrates the impact of different virus variants and respiratory PPE upon transmission risk. With the emergence of highly contagious SARS-CoV-2 variants such as the Delta and Omicron strains, respiratory PPE alone may not provide effective protection. These findings suggest a combination of optimal ventilation and respiratory PPE must be developed to effectively control the transmission of COVID-19 in healthcare settings and indoor spaces in general. This generalised risk estimation framework has the flexibility to incorporate further clinical data as such becomes available, and can be readily applied to consider a wide range of factors that impact transmission risk, including location and movement of infectious persons, virus variant and stage of infection, level of PPE and vaccination of infectious and susceptible individuals, impacts of coughing, sneezing, talking and breathing, and natural and mechanised ventilation and filtration.
Keywords: Covid-19; Delta variant; SARS-CoV-2; Spatiotemporal infection risk; Wells-Riley model.
Copyright © 2021 Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of competing interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures










References
-
- ANSI . vol. 55. American Society of Heating, Refrigerating and Air-Conditioning Engineers; 2004. Thermal Environmental Conditions for Human Occupancy.
-
- Carroll R.G. In: Elsevier's Integrated Physiology. Carroll R.G., editor. Mosby; Philadelphia: 2007. 10 - pulmonary system; pp. 99–115.
-
- Chen F., Yu S.C.M., Lai A.C.K. Modeling particle distribution and deposition in indoor environments with a new drift–flux model. Atmos. Environ. 2006;40:357–367.
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

