Rapid assessment of Opisthorchis viverrini IgG antibody in serum: A potential diagnostic biomarker to predict risk of cholangiocarcinoma in regions endemic for opisthorchiasis
- PMID: 34954313
- PMCID: PMC9569029
- DOI: 10.1016/j.ijid.2021.12.347
Rapid assessment of Opisthorchis viverrini IgG antibody in serum: A potential diagnostic biomarker to predict risk of cholangiocarcinoma in regions endemic for opisthorchiasis
Abstract
Background: Opisthorchiasis is caused by an infection with fish-borne liver flukes of the genus Opisthorchis. Opisthorchiasis frequently leads to chronic inflammation in the biliary tract and is classified as a group 1 biological carcinogen by the International Agency for Research on Cancer: a definitive risk for cholangiocarcinoma (CCA).
Methods: We used the rapid immunochromatographic test (ICT) to detect anti-Opisthorchis viverrini IgG and IgG4 subclass antibodies in sera of patients with CCA. The ICT kits were developed based on soluble antigens excreted and secreted by O. viverrini adult worms.
Results: ICT indicated sera was positive for IgG and IgG4 antibodies, respectively, in 22 (61.1%) and 15 (41.6%) participants of the 36 study participants diagnosed with CCA (P > 0.05). Our study also included groups with other cancers and with liver cirrhosis, where the IgG ICT and IgG4 ICT kits were 27.7% (13/47) and 25.5% (12/47) positive, respectively (P > 0.05). Neither total the IgG ICT nor the IgG4 ICT yielded positive results in a control group of 20 healthy participants. Moreover, the percentage positivity rate using the ICT for total IgG between the CCA group and the other cancers and liver cirrhosis group was significantly different (P < 0.05). By contrast, no significant difference between these groups was apparent in the ICT for IgG4 antibody. The CCA group was 6.53 times more likely to have positive anti-O. viverrini IgG antibody (odds ratio 6.53, P < 0.001) and 3.27 times more likely to have positive anti-O. viverrini IgG4 antibody (odds ratio 3.27, P = 0.010) than the non-CCA group.
Conclusion: This information is of potential value for the development of a diagnostic biomarker to predict risk for O. viverrini infection-associated CCA.
Keywords: Cholangiocarcinoma; Diagnostic biomarker; Helminthiases-associated cancer; Opisthorchiasis; Opisthorchis viverrini; Rapid serodiagnosis.
Copyright © 2021 The Author(s). Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
Conflicts of interest The authors declare that they have no conflicts of interest.
Figures
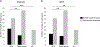
Similar articles
-
Detection of salivary antibodies to crude antigens of Opisthorchis viverrini in opisthorchiasis and cholangiocarcinoma patients.Clin Oral Investig. 2011 Aug;15(4):477-83. doi: 10.1007/s00784-010-0421-y. Epub 2010 May 6. Clin Oral Investig. 2011. PMID: 20446100
-
[Control of Opisthorchis viverrini infection for cholangiocarcinoma prevention].Bull Soc Pathol Exot. 2017 Feb;110(1):61-67. doi: 10.1007/s13149-017-0544-8. Epub 2017 Jan 19. Bull Soc Pathol Exot. 2017. PMID: 28105582 Review. French.
-
Comparative assessment of immunochromatographic test kits using somatic antigens from adult Opisthorchis viverrini and IgG and IgG4 conjugates for serodiagnosis of human opisthorchiasis.Parasitol Res. 2021 Aug;120(8):2839-2846. doi: 10.1007/s00436-021-07224-6. Epub 2021 Jul 14. Parasitol Res. 2021. PMID: 34259939 Free PMC article.
-
Specific serum IgG, but not IgA, antibody against purified Opisthorchis viverrini antigen associated with hepatobiliary disease and cholangiocarcinoma.Parasitol Int. 2012 Mar;61(1):212-6. doi: 10.1016/j.parint.2011.06.014. Epub 2011 Jun 23. Parasitol Int. 2012. PMID: 21718798
-
Diabetes mellitus: Possible risk and promoting factors of cholangiocarcinoma: Association of diabetes mellitus and cholangiocarcinoma.Cancer Epidemiol. 2015 Jun;39(3):274-8. doi: 10.1016/j.canep.2015.04.002. Epub 2015 Apr 22. Cancer Epidemiol. 2015. PMID: 25910864 Review.
Cited by
-
An Innovative Test for the Rapid Detection of Specific IgG Antibodies in Human Whole-Blood for the Diagnosis of Opisthorchis viverrini Infection.Trop Med Infect Dis. 2022 Oct 17;7(10):308. doi: 10.3390/tropicalmed7100308. Trop Med Infect Dis. 2022. PMID: 36288049 Free PMC article.
-
Immunomics-guided biomarker discovery for human liver fluke infection and infection-associated cholangiocarcinoma.Nat Commun. 2025 Jul 1;16(1):5965. doi: 10.1038/s41467-025-61043-2. Nat Commun. 2025. PMID: 40595543 Free PMC article.
-
LncRNA LUCAT1 as a prognostic biomarker in cholangiocarcinoma through targeting miR-141-3p: clinical and functional insights.Hereditas. 2025 Jul 26;162(1):143. doi: 10.1186/s41065-025-00512-6. Hereditas. 2025. PMID: 40713875 Free PMC article.
-
A first attempt at determining the antibody-specific pattern of Platynosomum fastosum crude antigen and identification of immunoreactive proteins for immunodiagnosis of feline platynosomiasis.Vet World. 2022 Aug;15(8):2029-2038. doi: 10.14202/vetworld.2022.2029-2038. Epub 2022 Aug 23. Vet World. 2022. PMID: 36313847 Free PMC article.
-
Current State of Knowledge on Blood and Tissue-Based Biomarkers for Opisthorchis viverrini-induced Cholangiocarcinoma: A Review of Prognostic, Predictive, and Diagnostic Markers.Asian Pac J Cancer Prev. 2024 Jan 1;25(1):25-41. doi: 10.31557/APJCP.2024.25.1.25. Asian Pac J Cancer Prev. 2024. PMID: 38285765 Free PMC article. Review.
References
-
- Banales JM, Cardinale V, Carpino G, Marzioni M, Andersen JB, Invernizzi P, et al. Expert consensus document: Cholangiocarcinoma: current knowledge and future perspectives consensus statement from the European Network for the Study of Cholangiocarcinoma (ENS-CCA). Nat Rev Gastroenterol Hepatol 2016;13:261–80. doi: 10.1038/nrgastro.2016.51. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

