Organoid and microfluidics-based platforms for drug screening in COVID-19
- PMID: 34954328
- PMCID: PMC8695520
- DOI: 10.1016/j.drudis.2021.12.014
Organoid and microfluidics-based platforms for drug screening in COVID-19
Abstract
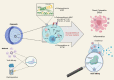
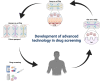
Proposing efficient prophylactic and therapeutic strategies for coronavirus 2019 (COVID-19) requires precise knowledge of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) pathogenesis. An array of platforms, including organoids and microfluidic devices, have provided a basis for studies of SARS-CoV-2. Here, we summarize available models as well as novel drug screening approaches, from simple to more advanced platforms. Notably, organoids and microfluidic devices offer promising perspectives for the clinical translation of basic science, such as screening therapeutics candidates. Overall, modifying these advanced micro and macro 3D platforms for disease modeling and combining them with recent advances in drug screening has significant potential for the discovery of novel potent drugs against COVID-19.
Keywords: COVID-19 modeling; Drug screening; Microfluidic technology; Organoid technology; SARS-CoV-2.
Copyright © 2021 Elsevier Ltd. All rights reserved.
Figures




Similar articles
-
Spheroids and organoids as humanized 3D scaffold-free engineered tissues for SARS-CoV-2 viral infection and drug screening.Artif Organs. 2021 Jun;45(6):548-558. doi: 10.1111/aor.13880. Epub 2021 Jan 10. Artif Organs. 2021. PMID: 33264436 Free PMC article. Review.
-
Human Organoids as a Promising Platform for Fighting COVID-19.Int J Biol Sci. 2022 Jan 1;18(3):901-910. doi: 10.7150/ijbs.64993. eCollection 2022. Int J Biol Sci. 2022. PMID: 35173525 Free PMC article. Review.
-
Insights into organoid-based modeling of COVID-19 pathology.Virol J. 2023 Feb 25;20(1):37. doi: 10.1186/s12985-023-01996-2. Virol J. 2023. PMID: 36841795 Free PMC article. Review.
-
Human organoid models to study SARS-CoV-2 infection.Nat Methods. 2022 Apr;19(4):418-428. doi: 10.1038/s41592-022-01453-y. Epub 2022 Apr 8. Nat Methods. 2022. PMID: 35396481 Review.
-
3D Engineering of Ocular Tissues for Disease Modeling and Drug Testing.Adv Exp Med Biol. 2019;1186:171-193. doi: 10.1007/978-3-030-28471-8_7. Adv Exp Med Biol. 2019. PMID: 31654390
Cited by
-
Nanosponges: An overlooked promising strategy to combat SARS-CoV-2.Drug Discov Today. 2022 Oct;27(10):103330. doi: 10.1016/j.drudis.2022.07.015. Epub 2022 Jul 28. Drug Discov Today. 2022. PMID: 35908684 Free PMC article. Review.
-
Organoids and organoid extracellular vesicles-based disease treatment strategies.J Nanobiotechnology. 2024 Nov 6;22(1):679. doi: 10.1186/s12951-024-02917-3. J Nanobiotechnology. 2024. PMID: 39506799 Free PMC article. Review.
-
A Shadow of Knowledge in Stem Cell Science.Cell J. 2023 Oct 1;25(10):738-740. doi: 10.22074/cellj.2023.2005680.1346. Cell J. 2023. PMID: 37865882 Free PMC article.
-
Aging on Chip: Harnessing the Potential of Microfluidic Technologies in Aging and Rejuvenation Research.Adv Healthc Mater. 2025 Aug;14(20):e2500217. doi: 10.1002/adhm.202500217. Epub 2025 Jun 12. Adv Healthc Mater. 2025. PMID: 40509615 Free PMC article. Review.
-
The Role of Biophysical Factors in Organ Development: Insights from Current Organoid Models.Bioengineering (Basel). 2024 Jun 18;11(6):619. doi: 10.3390/bioengineering11060619. Bioengineering (Basel). 2024. PMID: 38927855 Free PMC article. Review.
References
-
- Astashkina A., Mann B., Grainger D.W. A critical evaluation of in vitro cell culture models for high-throughput drug screening and toxicity. Pharmacol Ther. 2012;134:82–106. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Miscellaneous

