Computational modeling of mitochondrial K+- and H+-driven ATP synthesis
- PMID: 34954465
- PMCID: PMC8940703
- DOI: 10.1016/j.yjmcc.2021.12.005
Computational modeling of mitochondrial K+- and H+-driven ATP synthesis
Abstract
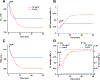
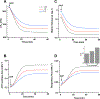
ATP synthase (F1Fo) is a rotary molecular engine that harnesses energy from electrochemical-gradients across the inner mitochondrial membrane for ATP synthesis. Despite the accepted tenet that F1Fo transports exclusively H+, our laboratory has demonstrated that, in addition to H+, F1Fo ATP synthase transports a significant fraction of ΔΨm-driven charge as K+ to synthesize ATP. Herein, we utilize a computational modeling approach as a proof of principle of the feasibility of the core mechanism underlying the enhanced ATP synthesis, and to explore its bioenergetic consequences. A minimal model comprising the 'core' mechanism constituted by ATP synthase, driven by both proton (PMF) and potassium motive force (KMF), respiratory chain, adenine nucleotide translocator, Pi carrier, and K+/H+ exchanger (KHEmito) was able to simulate enhanced ATP synthesis and respiratory fluxes determined experimentally with isolated heart mitochondria. This capacity of F1Fo ATP synthase confers mitochondria with a significant energetic advantage compared to K+ transport through a channel not linked to oxidative phosphorylation (OxPhos). The K+-cycling mechanism requires a KHEmito that exchanges matrix K+ for intermembrane space H+, leaving PMF as the overall driving energy of OxPhos, in full agreement with the standard chemiosmotic mechanism. Experimental data of state 4➔3 energetic transitions, mimicking low to high energy demand, could be reproduced by an integrated computational model of mitochondrial function that incorporates the 'core' mechanism. Model simulations display similar behavior compared to the experimentally observed changes in ΔΨm, mitochondrial K+ uptake, matrix volume, respiration, and ATP synthesis during the energetic transitions at physiological pH and K+ concentration. The model also explores the role played by KHEmito in modulating the energetic performance of mitochondria. The results obtained support the available experimental evidence on ATP synthesis driven by K+ and H+ transport through the F1Fo ATP synthase.
Keywords: Energy supply-demand matching; F(1)F(o) ATP synthase; Mitochondrial K(+) uptake; Mitochondrial K(+)/H(+) exchanger.
Copyright © 2021. Published by Elsevier Ltd.
Figures




References
-
- Mitchell P, Coupling of phosphorylation to electron and hydrogen transfer by a chemi-osmotic type of mechanism, Nature 191 (1961) 144–8. - PubMed
-
- Walker JE, The ATP synthase: the understood, the uncertain and the unknown, Biochem Soc Trans 41(1) (2013) 1–16. - PubMed
-
- Juhaszova M, Kobrinsky E, Zorov DB, Nuss HB, Yaniv Y, Fishbein KW, et al. , ATP synthase K+- and H+-flux drive ATP synthesis and enable mitochondrial K+-uniporter function, BioRxiv 355776 (2019).
-
- Kobrinsky E, Zorov DB, Juhaszova M, Aon MA, Cortassa S, Sollott SJ, Mitochondrial ATP Synthase Utilizes Both K+ and H+ Conductances to Drive ATP Synthesis, Biophysical Journal 118(3) (2020) 441a–441a.
-
- Nichols DG, Ferguson SJ, Bioenergetics, Fourth ed., Academic Press, London, U.K., 2013.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

