Mucosal immune response in BNT162b2 COVID-19 vaccine recipients
- PMID: 34954658
- PMCID: PMC8718969
- DOI: 10.1016/j.ebiom.2021.103788
Mucosal immune response in BNT162b2 COVID-19 vaccine recipients
Abstract
Background: Although the BNT162b2 COVID-19 vaccine is known to induce IgG neutralizing antibodies in serum protecting against COVID-19, it has not been studied in detail whether it could generate specific immunity at mucosal sites, which represent the primary route of entry of SARS-CoV-2.
Methods: Samples of serum and saliva of 60 BNT162b2-vaccinated healthcare workers were collected at baseline, two weeks after the first dose and two weeks after the second dose. Anti-S1-protein IgG and IgA total antibodies titres and the presence of neutralizing antibodies against the Receptor Binding Domain in both serum and saliva were measured by quantitative and by competitive ELISA, respectively.
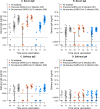
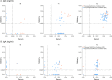
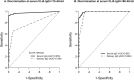
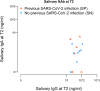
Findings: Complete vaccination cycle generates a high serum IgG antibody titre as a single dose in previously infected seropositive individuals. Serum IgA concentration reaches a plateau after a single dose in seropositive individuals and two vaccine doses in seronegative subjects. After the second dose IgA level was higher in seronegative than in seropositive subjects. In saliva, IgG level is almost two orders of magnitude lower than in serum, reaching the highest values after the second dose. IgA concentration remains low and increases significantly only in seropositive individuals after the second dose. Neutralizing antibody titres were much higher in serum than in saliva.
Interpretation: The mRNA BNT162b2 vaccination elicits a strong systemic immune response by drastically boosting neutralizing antibodies development in serum, but not in saliva, indicating that at least oral mucosal immunity is poorly activated by this vaccination protocol, thus failing in limiting virus acquisition upon its entry through this route.
Funding: This work was funded by the Department of Medicine and Surgery, University of Insubria, and partially supported by Fondazione Umberto Veronesi (COVID-19 Insieme per la ricerca di tutti, 2020).
Keywords: BNT162b2 mRNA vaccine; COVID-19; IgA; SARS-CoV-2; Saliva.
Copyright © 2021 The Author(s). Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of interests None to declare.
Figures
Comment in
-
Importance of nasal secretions in the evaluation of mucosal immunity elicited by mRNA BNT162b2 COVID-19 Vaccine.EBioMedicine. 2022 May;79:104006. doi: 10.1016/j.ebiom.2022.104006. Epub 2022 Apr 14. EBioMedicine. 2022. PMID: 35430452 Free PMC article. No abstract available.
References
-
- World Health Organization . 2021. Coronavirus disease (COVID-19) dashboard.https://covid19.who.int accessed on December 5th, 2021. - PubMed
-
- Lavezzo E, Franchin E, Ciavarella C, et al. Suppression of a SARS-CoV-2 outbreak in the Italian municipality of Vo’. Nature. 2020;584:425–429. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

