Randomized Clinical Trial of Endovascular Therapy for Acute Large Vessel Occlusion with Large Ischemic Core (RESCUE-Japan LIMIT): Rationale and Study Protocol
- PMID: 34955488
- PMCID: PMC8918370
- DOI: 10.2176/nmc.rc.2021-0311
Randomized Clinical Trial of Endovascular Therapy for Acute Large Vessel Occlusion with Large Ischemic Core (RESCUE-Japan LIMIT): Rationale and Study Protocol
Abstract
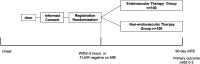
Endovascular therapy is strongly recommended for acute cerebral large vessel occlusion (LVO) with an Alberta stroke program early computed tomography score (ASPECTS) ≥6 due to occlusion of the internal carotid artery or M1 segment of the middle cerebral artery. However, the effect of endovascular therapy for patients with a large ischemic core with an ASPECTS ≤5 (0-5) was not established. A multicenter, randomized, open-label, parallel-group trial was conducted to investigate the superiority of endovascular therapy over medical therapy without endovascular therapy for a large ischemic core with ASPECTS (3-5). Patients were randomly assigned to receive endovascular therapy or without endovascular therapy at a ratio of 1:1. The primary outcome was a moderate functional outcome, defined as a modified Rankin scale (mRS; scores ranging from 0 [no symptoms] to 6 [death]) ≤3 after 90 days. The secondary outcomes were defined as ordinal mRS, good functional outcome (mRS ≤2), excellent functional outcome (mRS ≤1), mRS shift analysis after 90 days, and early improvement of neurological findings at 48 hours. A total sample size of 200 was estimated to provide a power of 0.9 with a two-sided alpha of 0.05, for the primary outcome, considering a 15% dropout rate. This randomized clinical trial reported the applicability of endovascular therapy in patients with acute cerebral LVO with a large ischemic core.
Keywords: acute ischemic stroke; endovascular therapy; large ischemic core; large vessel occlusion; randomized clinical trial.
Conflict of interest statement
Dr. Yoshimura reports research grants from Medico’s Hirata, Medtronic, and Termo, and lecturer fees from Medtronic, Kaneka, and Stryker. Dr. Uchida reports lecturer’s fees from Daiichi-Sankyo, Bristol-Myers Squibb, Stryker, and Medtronic. Dr. Sakai reports a research grant from Biomedical Solutions, Daiichi-Sankyo, and Termo; lecturer’s fees from Asahi-Intec, Biomedical Solutions, Daiichi-Sankyo, and Medtronic; and membership on the advisory boards for Johnson & Johnson, Medtronic, and Terumo. Dr. Yamagami discloses research grants from Bristol-Myers Squibb; lecturer’s fees from Stryker, Medtronic, Termo, Johnson & Johnson, Biomedical Solutions, and Medico’s Hirata; and membership of the advisory boards for Daiichi-Sankyo. Dr. Inoue reports the lecturer’s fees from Bayer Co., Ltd., Bristol-Myers Squibb Co., Ltd., Medico’s Hirata Co., Ltd.; and manuscript fees from Gakken Medical Co., Ltd. and Hokuryukan Co., Ltd. Dr. Toyoda reports the lecturer’s fees from Daiichi Sankyo, Takeda, Bayer Yakuhin, and Bristol-Myers Squibb. Dr. Matsumaru discloses lecturer fees from Medtronic, Stryker, Terumo, Johnson & Johnson, Kaneka, and Jimro. Dr. Mastumoto reports the lecturer’s fees from Kaneka Medics, Medico’s Hirata, Fuji systems, GE healthcare, Otuka Pharmaceutical Limited, Takeda Pharmaceutical Limited, Medtronic Japan, Century Medical, Terumo, Medtronic, and Stryker Japan. Dr. Kimura discloses research grants from CSL Behring K.K., EP-CRSU Co., Ltd., AABP K.K., Alexion Pharmaceuticals, Inc., Eisai Co., Ltd., Kyowa Kirin Co., Ltd., Daiichi-Sankyo Company, Ltd., Teijin Pharma Ltd., Medtronic Japan Co., Ltd., Bristol-Myers Squibb K.K., Bayer Yakuhin, Ltd., Boehringer-Ingelheim, and Helios Co., Ltd., and lecturer’s fees from Daiichi-Sankyo Company, Ltd., Nippon Boehringer Ingelheim Co., Ltd., Bristol-Myers Squibb K.K., Bayer Yakuhin, Ltd., Takeda Pharmaceutical Co., Ltd., Medtronic Japan Co., Ltd., Otsuka Pharmaceutical Co., Ltd., FP Pharmaceutical Co., Ltd., Alexion Pharmaceuticals, Inc., Nippon Pharmaceutical Co., Ltd., Chugai Pharmaceutical Co., Ltd., Kyowa Kirin Co., Ltd., Abbott Medical Japan LLC, Shire PLC, Sanofi Genzyme K.K., CSL Behring K.K., Novartis Pharma K.K., Toa Eiyo Ltd., Medico’s Hirata Inc., and Helios Co., Ltd. Dr. Morimoto reports lecturers’ fees from Bristol-Myers Squibb, Daiichi Sankyo, Japan Lifeline, Kowa, Kyocera, Novartis, and Toray; manuscript fees from Bristol-Myers Squibb and Kowa; and advisory board for Sanofi. Dr. Ishikura has no conflicts of interest to report.
Figures
References
-
- Powers WJ, Rabinstein AA, Ackerson T, et al. : Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 guidelines for the early management of acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 50: e344–e418, 2019 - PubMed
-
- Yamagami H, Hayakawa M, Inoue M, et al. : Guidelines for mechanical thrombectomy in Japan, the Fourth Edition, March 2020: a guideline from the Japan Stroke Society, the Japan Neurosurgical Society, and the Japanese Society for Neuroendovascular Therapy. Neurol Med Chir (Tokyo) 61: 163–192, 2021 - PMC - PubMed
-
- Cucchiara B, Kasner SE, Tanne D, et al. : Factors associated with intracerebral hemorrhage after thrombolytic therapy for ischemic stroke: pooled analysis of placebo data from the Stroke-Acute Ischemic NXY Treatment (SAINT) I and SAINT II Trials. Stroke 40: 3067–3072, 2009 - PubMed
-
- Berkhemer OA, Fransen PS, Beumer D, et al. : A randomized trial of intraarterial treatment for acute ischemic stroke. N Engl J Med 372: 11–20, 2015 - PubMed

