In situ arsenic immobilisation for coastal aquifers using stimulated iron cycling: Lab-based viability assessment
- PMID: 34955596
- PMCID: PMC8699153
- DOI: 10.1016/j.apgeochem.2021.105155
In situ arsenic immobilisation for coastal aquifers using stimulated iron cycling: Lab-based viability assessment
Abstract
Arsenic (As) is one of the most harmful and widespread groundwater contaminants globally. Besides the occurrence of geogenic As pollution, there is also a large number of sites that have been polluted by anthropogenic activities, with many of those requiring active remediation to reduce their environmental impact. Cost-effective remedial strategies are however still sorely needed. At the laboratory-scale in situ formation of magnetite through the joint addition of nitrate and Fe(II) has shown to be a promising new technique. However, its applicability under a wider range of environmental conditions still needs to be assessed. Here we use sediment and groundwater from a severely polluted coastal aquifer and explore the efficiency of nitrate-Fe(II) treatments in mitigating dissolved As concentrations. In selected experiments >99% of dissolved As was removed, compared to unamended controls, and maintained upon addition of lactate, a labile organic carbon source. Pre- and post experimental characterisation of iron (Fe) mineral phases suggested a >90% loss of amorphous Fe oxides in favour of increased crystalline, recalcitrant oxide and sulfide phases. Magnetite formation did not occur via the nitrate-dependent oxidation of the amended Fe(II) as originally expected. Instead, magnetite is thought to have formed by the Fe(II)-catalysed transformation of pre-existing amorphous and crystalline Fe oxides. The extent of amorphous and crystalline Fe oxide transformation was then limited by the exhaustion of dissolved Fe(II). Elevated phosphate concentrations lowered the treatment efficacy indicating joint removal of phosphate is necessary for maximum impact. The remedial efficiency was not impacted by varying salinities, thus rendering the tested approach a viable remediation method for coastal aquifers.
Keywords: arsenic remediation; bioremediation; coastal aquifer; in situ mineral precipitation.
Conflict of interest statement
Declaration of interests The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures






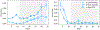

References
-
- APHA, A.P.H.A., 1995. Standard Methods For the Examination of Water and Wastewater, 23nd edition, Standard Methods For the Examination of Water and Wastewater, 19th edition. New York. 10.2105/SMWW.2882.023 - DOI
-
- ATSDR, A. for T.S. and D.R., 2019. Substance Priority List Resource Page | ATSDR.
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
