Toward a Practical, Nonenzymatic Process for Investigational COVID-19 Antiviral Molnupiravir from Cytidine: Supply-Centered Synthesis
- PMID: 34955627
- PMCID: PMC8689649
- DOI: 10.1021/acs.oprd.1c00219
Toward a Practical, Nonenzymatic Process for Investigational COVID-19 Antiviral Molnupiravir from Cytidine: Supply-Centered Synthesis
Abstract
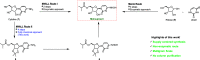
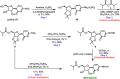
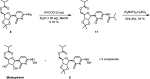
A scalable four-step synthesis of molnupiravir from cytidine is described herein. The attractiveness of this approach is its fully chemical nature involving inexpensive reagents and more environmentally friendly solvents such as water, isopropanol, acetonitrile, and acetone. Isolation and purification procedures are improved in comparison to our earlier study as all intermediates can be isolated via recrystallization. The key steps in the synthesis, namely, ester formation, hydroxyamination, and deprotection were carried out on a multigram scale to afford molnupiravir in 36-41% yield with an average purity of 98 wt % by qNMR and 99 area% by HPLC.
© 2021 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


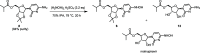
References
-
-
Recent news articles see
- Kropff A. “Coronavirus” https://www.wtsp.com/article/news/health/coronavirus/antiviral-drug-moln..., Published Mar 26, 2021.
- Merck media “News release”. https://www.merck.com/news/merck-and-ridgeback-biotherapeutics-provide-u...
-
-
- Sheahan T. P.; Sims A. C.; Zhou S.; Graham R. L.; Pruijssers A. J.; Agostini M. L.; Leist S. R.; Schäfer A.; Dinnon K. H. III; Stevens L. J.; Chappell J. D.; Lu X.; Hughes T. M.; George A. S.; Hill C. S.; Montgomery S. A.; Brown A. J.; Bluemling G. R.; Natchus M. G.; Saindane M.; Kolykhalov A. A.; Painter G.; Harcourt J.; Tamin A.; Thornburg N. J.; Swanstrom R.; Denison M. R.; Baric R. S. An orally bioavailable broad-spectrum antiviral inhibits SARS-CoV-2 in human airway epithelial cell cultures and multiple coronaviruses in mice. Sci. Transl. Med. 2020, 12, 541.10.1126/scitranslmed.abb5883. - DOI - PMC - PubMed
-
- Painter G. R.; Perryman D.; Bluemling G. R.. 4’-Halogen Containing Nucleotide and Nucleoside Therapeutic Compositions and Uses Related Thereto, G.R.WO2019173602 September 12, 2019.
- Painter G.; Bluemling G.; Natchus M.; Guthrie D.. N4-Hydroxycytidine and Derivatives and Anti-Viral Uses Related Thereto, WO2019113462, June 13, 2019.
-
- Vasudevan N.; Ahlqvist G. P.; McGeough C. P.; Paymode D. J.; Cardoso F. S. P.; Lucas T.; Dietz J.-P.; Opatz T.; Jamison T. F.; Gupton F. B.; Snead D. R. A Concise Route to MK-4482 (EIDD-2801) from Cytidine. Chem. Commun. 2020, 56, 13363–13364. 10.1039/D0CC05944G. - DOI - PubMed
- Gopalsamuthiram V.; Williams C.; Noble J.; Jamison T. F.; Gupton B. F.; Snead D. R. A Concise Route to MK-4482 (EIDD-2801) from Cytidine: Part 2. Synlett 2021, 32, 326–328. 10.1055/a-1275-2848. - DOI
- Ahlqvist G. P.; McGeough C. P.; Senanayake C.; Armstrong J. D.; Yadaw A.; Roy S.; Ahmad S.; Snead D. R.; Jamison T. F. Progress Toward a Large-Scale Synthesis of Molnupiravir (MK-4482, EIDD-2801) from Cytidine. ACS Omega 2021, 6, 10396–10402. 10.1021/acsomega.1c00772. - DOI - PMC - PubMed
- Paymode D. J.; Vasudevan N.; Ahmad S.; Kadam A. L.; Cardoso F. S. P.; Burns J. M.; Cook D. W.; Stringham R. W.; Snead D. R. Toward a Practical, Two-Step Process for Molnupiravir: Direct Hydroxyamination of Cytidine Followed by Selective Esterification. Org. Process Res. Dev. 2021, 25, 1822–1830. 10.1021/acs.oprd.1c00033. - DOI
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
