Expression and Function of ABC Proteins in Fish Intestine
- PMID: 34955897
- PMCID: PMC8696203
- DOI: 10.3389/fphys.2021.791834
Expression and Function of ABC Proteins in Fish Intestine
Abstract
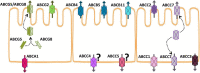
In fish, the intestine is fundamental for digestion, nutrient absorption, and other functions like osmoregulation, acid-base balance, and excretion of some metabolic products. These functions require a large exchange surface area, which, in turn, favors the absorption of natural and anthropogenic foreign substances (xenobiotics) either dissolved in water or contained in the food. According to their chemical nature, nutrients, ions, and water may cross the intestine epithelium cells' apical and basolateral membranes by passive diffusion or through a wide array of transport proteins and also through endocytosis and exocytosis. In the same way, xenobiotics can cross this barrier by passive diffusion or taking advantage of proteins that transport physiological substrates. The entry of toxic substances is counterbalanced by an active efflux transport mediated by diverse membrane proteins, including the ATP binding cassette (ABC) proteins. Recent advances in structure, molecular properties, and functional studies have shed light on the importance of these proteins in cellular and organismal homeostasis. There is abundant literature on mammalian ABC proteins, while the studies on ABC functions in fish have mainly focused on the liver and, to a minor degree, on the kidney and other organs. Despite their critical importance in normal physiology and as a barrier to prevent xenobiotics incorporation, fish intestine's ABC transporters have received much less attention. All the ABC subfamilies are present in the fish intestine, although their functionality is still scarcely studied. For example, there are few studies of ABC-mediated transport made with polarized intestinal preparations. Thus, only a few works discriminate apical from basolateral transport activity. We briefly describe the main functions of each ABC subfamily reported for mammals and other fish organs to help understand their roles in the fish intestine. Our study considers immunohistochemical, histological, biochemical, molecular, physiological, and toxicological aspects of fish intestinal ABC proteins. We focus on the most extensively studied fish ABC proteins (subfamilies ABCB, ABCC, and ABCG), considering their apical or basolateral location and distribution along the intestine. We also discuss the implication of fish intestinal ABC proteins in the transport of physiological substrates and aquatic pollutants, such as pesticides, cyanotoxins, metals, hydrocarbons, and pharmaceutical products.
Keywords: aquatic pollutants; detoxification; epithelial physiology; multixenobiotic resistance; polarized transport.
Copyright © 2021 Bieczynski, Painefilú, Venturino and Luquet.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
Publication types
LinkOut - more resources
Full Text Sources

