Erenumab for Migraine Prevention in a 1-Year Compassionate Use Program: Efficacy, Tolerability, and Differences Between Clinical Phenotypes
- PMID: 34956071
- PMCID: PMC8703164
- DOI: 10.3389/fneur.2021.805334
Erenumab for Migraine Prevention in a 1-Year Compassionate Use Program: Efficacy, Tolerability, and Differences Between Clinical Phenotypes
Abstract
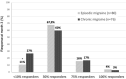
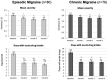
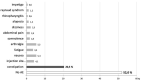
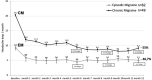
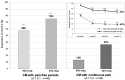
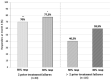
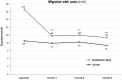
During a 1-year compassionate use program, 156 patients with migraine self-administered a monthly dose of erenumab 140 mg with a subcutaneous autoinjector. Main inclusion criteria were: ≥ 4 migraine days/month and ≥two prior prophylactic treatment failures. The patients covered the migraine severity spectrum from episodic migraine (EM) (n = 80) to chronic migraine (CM) (n = 76). During the 3rd month of treatment, monthly headache days decreased by 45.7% in EM and 35.5% in CM. The 50% responder rate for reduction in monthly headache days was significantly higher in EM (55%) than in CM (43%) (p = 0.05). In both the migraine subgroups, the clinical improvement vs. baseline was already significant during the 1st month of treatment (p < 0.001). There were also significant reductions in mean headache severity, duration, and monthly days with acute drug intake. The 30% responder rate at 3 months was 60% in CM and 54.1% of patients reversed from CM to EM. The therapeutic effect was maintained at 12 months when 50% responder rates, considering discontinuation for lack of efficacy or adverse effects as 0% response, still were 51% in EM and 41% in CM. A total of 10 patients with EM (12.5%) and 23 patients with CM (30.3%) had discontinued treatment, considering the treatment as ineffective. At 3 months, 48% of patients reported non-serious adverse events among which the most frequent was constipation (20.5%); corresponding figures at 12 months were 30 and 15%. Discontinuation due to an adverse effect for the entire 12 month period was rare (3.8%). The lower efficacy in CM than in EM was mainly due to a very low 50% responder rate in patients with CM with continuous pain (13%) as compared to CM with pain-free periods (58%) (p < 0.001). Similarly, the 50% responder rate was lower in patients with ≥two prior prophylactic treatment failures (40.5%) compared to those with two failures (70%) (p < 0.05). There was no significant efficacy difference between low (4-7 migraine days/month, n = 22) and high frequency (8-14 days, n = 59) EM nor between patients with CM with (n = 50) or without (n = 26) acute medication overuse. Erenumab had no effect on the frequency of auras. Taken together, erenumab 140 mg monthly was highly effective for migraine prophylaxis over the whole severity spectrum of the disease, except in patients with continuous headaches. Its effect is significant after the first injection, quasi-maximal after the second injection, and does not wear off after 12 months. The most frequent adverse effect was constipation. These results are compared to those published for erenumab in the pivotal randomized placebo-controlled trials and to those reported in several recent real-world studies.
Keywords: compassionate use; erenumab; migraine prophylaxis; monoclonal antibodies blocking CGRP transmission; outcome predictors.
Copyright © 2021 Schoenen, Timmermans, Nonis, Manise, Fumal and Gérard.
Conflict of interest statement
JS is an advisor for Novartis Benelux, Teva Pharma, Lundbeck and Man & Science; he has been a principal investigator in clinical trials sponsored by Novartis, Teva and Eli Lilly. GT has been an investigator in clinical trials sponsored by Novartis, Teva and Eli Lilly. RN and PG have been data managers in clinical trials sponsored by Novartis, Teva and Eli Lilly. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
LinkOut - more resources
Full Text Sources
Medical
Research Materials

