Effect of Different Disease-Modifying Therapies on Humoral Response to BNT162b2 Vaccine in Sardinian Multiple Sclerosis Patients
- PMID: 34956211
- PMCID: PMC8697018
- DOI: 10.3389/fimmu.2021.781843
Effect of Different Disease-Modifying Therapies on Humoral Response to BNT162b2 Vaccine in Sardinian Multiple Sclerosis Patients
Abstract
Objectives: Vaccination against COVID-19 is highly recommended to patients affected by multiple sclerosis (MS); however, the impact of MS disease-modifying therapies (DMTs) on the immune response following vaccination has been only partially investigated. Here, we aimed to elucidate the effect of DMTs on the humoral immune response to mRNA-based anti-SARS-CoV-2 vaccines in MS patients.
Methods: We obtained sera from 912 Sardinian MS patients and 63 healthy controls 30 days after the second dose of BNT162b2 vaccine and tested them for SARS-CoV-2 response using anti-Spike (S) protein-based serology. Previous SARS-CoV-2 infection was assessed by anti-Nucleocapsid (N) serology. Patients were either untreated or undergoing treatment with a total of 13 different DMTs. Differences between treatment groups comprised of at least 10 patients were assessed by generalized linear mixed-effects model. Demographic and clinical data and smoking status were analyzed as additional factors potentially influencing humoral immunity from COVID-19 vaccine.
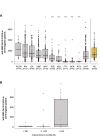
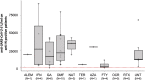
Results: MS patients treated with natalizumab, teriflunomide, azathioprine, fingolimod, ocrelizumab, and rituximab showed significantly lower humoral responses compared to untreated patients. We did not observe a statistically significant difference in response between patients treated with the other drugs (dimethyl fumarate, interferon, alemtuzumab and glatiramer acetate) and untreated patients. In addition, older age, male sex and active smoking were significantly associated with lower antibody titers against SARS-CoV-2. MS patients previously infected with SARS-CoV-2 had significantly higher humoral responses to vaccine than uninfected patients.
Conclusion: Humoral response to BNT162b2 is significantly influenced by the specific DMTs followed by patients, as well as by other factors such as previous SARS-CoV-2 infection, age, sex, and smoking status. These results are important to inform targeted strategies to prevent clinically relevant COVID-19 in MS patients.
Keywords: BNT162b2; COVID-19; SARS-CoV-2; disease-modifying therapy; humoral immunity; multiple sclerosis; vaccine efficacy.
Copyright © 2021 Pitzalis, Idda, Lodde, Loizedda, Lobina, Zoledziewska, Virdis, Delogu, Pirinu, Marini, Mingoia, Frau, Lorefice, Fronza, Carmagnini, Carta, Orrù, Uzzau, Solla, Loi, Devoto, Steri, Fiorillo, Floris, Zarbo, Cocco and Cucca.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Comment in
-
Response to SARS-CoV-2 vaccination in multiple sclerosis patients on disease modifying therapies.J Neurol. 2022 Apr;269(4):2259-2261. doi: 10.1007/s00415-022-11053-7. Epub 2022 Mar 14. J Neurol. 2022. PMID: 35286479 Free PMC article. No abstract available.
References
-
- Schultheiß C, Paschold L, Simnica D, Mohme M, Willscher E, von Wenserski L, et al. . Next-Generation Sequencing of T and B Cell Receptor Repertoires From COVID-19 Patients Showed Signatures Associated With Severity of Disease. Immunity (2020) 53:442–455.e4. doi: 10.1016/j.immuni.2020.06.024 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

