SARS-CoV-2 Antibodies Are Persisting in Saliva for More Than 15 Months After Infection and Become Strongly Boosted After Vaccination
- PMID: 34956236
- PMCID: PMC8695841
- DOI: 10.3389/fimmu.2021.798859
SARS-CoV-2 Antibodies Are Persisting in Saliva for More Than 15 Months After Infection and Become Strongly Boosted After Vaccination
Abstract
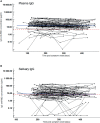
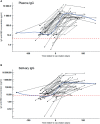
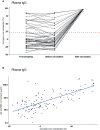
SARS-CoV-2 antibodies in saliva serve as first line of defense against the virus. They are present in the mucosa, more precisely in saliva, after a recovered infection and also following vaccination. We report here the antibody persistence in plasma and in saliva up to 15 months after mild COVID-19. The IgG antibody response was measured every two months in 72 participants using an established and validated in-house ELISA assay. In addition, the virus inhibitory activity of plasma antibodies was assessed in a surrogate virus neutralization test before and after vaccination. SARS-CoV-2-specific antibody concentrations remained stable in plasma and saliva and the response was strongly boosted after one dose COVID-19 vaccination.
Keywords: COVID-19; SARS-CoV-2; antibody; convalescent; mucosa; neutralization; saliva; vaccination.
Copyright © 2021 Pinilla, Heinzel, Caminada, Consolaro, Esen, Kremsner, Held, Kreidenweiss and Fendel.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- Hansen CH, Michlmayr D, Gubbels SM, Mølbak K, Ethelberg S. Assessment of Protection Against Reinfection With SARS-CoV-2 Among 4 Million PCR-Tested Individuals in Denmark in 2020: A Population-Level Observational Study. Lancet (2021) 397(10280):1204–12. doi: 10.1016/S0140-6736(21)00575-4 - DOI - PMC - PubMed
-
- Pilgrim MJ, Kasman L, Grewal J, Bruorton ME, Werner P, London L, et al. . A Focused Salivary Gland Infection With Attenuated MCMV: An Animal Model With Prevention of Pathology Associated With Systemic MCMV Infection. Exp Mol Pathol (2007) 82(3):269–79. doi: 10.1016/j.yexmp.2006.12.010 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

