Intraparticle Kinetics Unveil Crowding and Enzyme Distribution Effects on the Performance of Cofactor-Dependent Heterogeneous Biocatalysts
- PMID: 34956691
- PMCID: PMC8689653
- DOI: 10.1021/acscatal.1c03760
Intraparticle Kinetics Unveil Crowding and Enzyme Distribution Effects on the Performance of Cofactor-Dependent Heterogeneous Biocatalysts
Abstract
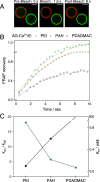
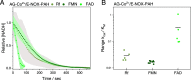
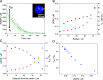
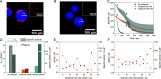
Multidimensional kinetic analysis of immobilized enzymes is essential to understand the enzyme functionality at the interface with solid materials. However, spatiotemporal kinetic characterization of heterogeneous biocatalysts on a microscopic level and under operando conditions has been rarely approached. As a case study, we selected self-sufficient heterogeneous biocatalysts where His-tagged cofactor-dependent enzymes (dehydrogenases, transaminases, and oxidases) are co-immobilized with their corresponding phosphorylated cofactors [nicotinamide adenine dinucleotide phosphate (NAD(P)H), pyridoxal phosphate (PLP), and flavin adenine dinucleotide (FAD)] on porous agarose microbeads coated with cationic polymers. These self-sufficient systems do not require the addition of exogenous cofactors to function, thus avoiding the extensive use of expensive cofactors. To comprehend the microscopic kinetics and thermodynamics of self-sufficient systems, we performed fluorescence recovery after photobleaching measurements, time-lapse fluorescence microscopy, and image analytics at both single-particle and intraparticle levels. These studies reveal a thermodynamic equilibrium that rules out the reversible interactions between the adsorbed phosphorylated cofactors and the polycations within the pores of the carriers, enabling the confined cofactors to access the active sites of the immobilized enzymes. Furthermore, this work unveils the relationship between the apparent Michaelis-Menten kinetic parameters and the enzyme density in the confined space, eliciting a negative effect of molecular crowding on the performance of some enzymes. Finally, we demonstrate that the intraparticle apparent enzyme kinetics are significantly affected by the enzyme spatial organization. Hence, multiscale characterization of immobilized enzymes serves as an instrumental tool to better understand the in operando functionality of enzymes within confined spaces.
© 2021 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures




References
-
- Guisan J. M.; Bolivar J. M.; López-gallego F.. Immobilization of Enzymes and Cells; Guisan J. M.; Bolivar J. M.; López-Gallego F.; Rocha-Martín J., Eds.; Methods in Molecular Biology; Springer US: New York, NY, 2020; Vol. 2100. - PubMed
-
- Brena B.; González-Pombo P.; Batista-Viera F.. Immobilization of Enzymes: A Literature Survey. In Immobilization of Enzymes and Cells, 3rd ed.; Guisan J. M., Ed.; Methods in Molecular Biology; Humana Press: Totowa, NJ, 2013; Vol. 1051, pp 15–31. - PubMed
-
- Garcia-Galan C.; Berenguer-Murcia Á.; Fernandez-Lafuente R.; Rodrigues R. C. Potential of Different Enzyme Immobilization Strategies to Improve Enzyme Performance. Adv. Synth. Catal. 2011, 353, 2885–2904. 10.1002/adsc.201100534. - DOI
LinkOut - more resources
Full Text Sources
Research Materials
