Update on Retinal Drug Toxicities
- PMID: 34956737
- PMCID: PMC8688906
- DOI: 10.1007/s40135-021-00277-x
Update on Retinal Drug Toxicities
Abstract
Purpose of review: This review aims to provide an update on the clinical presentations and diagnostic findings of drug-induced retinal toxicities.
Recent findings: Several newly FDA-approved medications have been associated with acute retinal toxicities, including brolucizumab, MEK inhibitors, ulixertinib, and FGFR inhibitors. Additionally, as previously believed-to-be well-tolerated medications, such as pentosan sulfate sodium, anti-retroviral therapies, and certain intraoperative ocular medications, are used more frequently or for longer periods of time, associated toxic retinopathies and inflammatory reactions have been reported. Finally, advances in ocular imaging have revealed novel findings in hydroxychloroquine and tamoxifen maculopathies.
Summary: Discovery of new medications, increased frequency of use, and longer-term use have led to increased reports of retinal toxicities. Advances in retinal imaging have allowed for earlier detection of subclinical changes associated with these medications, which may help prevent progression of disease. However, more research is needed to determine the point at which vision loss becomes irreversible. Risks and benefits must be assessed prior to discontinuation of the offending, but potentially lifesaving, therapy.
Keywords: Drug-induced maculopathy; Drug-induced retinopathy; Hemorrhagic occlusive retinal vasculitis, Brolucizumab, Pentosan sulfate; Retinal toxicity; Toxic retinopathy.
© The Author(s), under exclusive licence to Springer Science+Business Media, LLC, part of Springer Nature 2021.
Conflict of interest statement
Conflict of InterestNone of the authors has disclosures related to the submitted work. Dr. Deaner has the following disclosures outside of the submitted work: Alimera Sciences, Inc.: honoraria. Dr. Vajzovic has the following disclosures outside of the submitted work: Applied Genetic Technologies Corporation (AGTC): investigator; Alcon: investigator, consultant; Aldeyra: investigator; Alimera Sciences: consultant; Allergan — consultant; Aerie: consultant; Baush & Lomb: consultant; Beaver-Visitec International (BVI): consultant; Dutch Ophthalmic Research Center (DORC): consultant; Guidepoint: consultant; Gyroscope/Orbit Biomedical — research grant, consultant; Heidelberg Engineering — investigator, research grant; Janssen Pharmaceutical: consultant; Novartis: investigator, consultant; Oculus Surgical: consultant; Regenxbio: investigator; Roche/Genentech — investigator, consultant; Second Sight — investigator, consultant; Evolve Medical Education: honoraria.
Figures
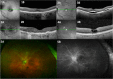
References
-
- Romano MR, Raimondi R, Montericcio A, Allegrini D. Hydroxychloroquine and ritonavir for COVID-19 infection: a possible synergic toxicity for retinal pigmented epithelium. Graefe’s Archive for Clinical and Experimental Ophthalmology. 2020;258(12):2871. doi: 10.1007/s00417-020-04727-3. - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
