Case Report: Graft Versus Tumor Effect After Non-Myeloablative Allogeneic Stem-Cell Transplantation in a Patient With Brentuximab-Vedotin Refractory Sezary Syndrome
- PMID: 34956873
- PMCID: PMC8695846
- DOI: 10.3389/fonc.2021.749691
Case Report: Graft Versus Tumor Effect After Non-Myeloablative Allogeneic Stem-Cell Transplantation in a Patient With Brentuximab-Vedotin Refractory Sezary Syndrome
Abstract
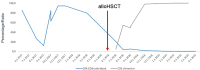
Sezary Syndrome (SS) is a rare leukemic variant of primary cutaneous T-cell lymphoma. Relapsed or refractory disease is generally considered incurable by conventional therapeutic approaches, although durable responses can be achieved with novel monoclonal antibodies. Allogeneic hematopoietic stem cell transplantation (alloHSCT) may have potential value by inducing graft vs-lymphoma (GvL) effects, but there is currently no consensus regarding the timing of alloHSCT or type of conditioning regimen. Here we present the case of a male patient who achieved a complete remission (CR) of primary refractory SS after non-myeloablative alloHSCT. Patient: Two years prior to HSCT, the patient had been refractory to CHOEP-based chemotherapy, interferon, extracorporeal photopheresis (ECP), and bexarotene. Directly prior to alloHSCT brentuximab-vedotin (BV) was applied resulting in a partial remission of the skin compartment and overall in a stable disease. Prior to HSCT, flow cytometry of the bone marrow and peripheral blood showed an infiltration with T-cells positive for CD5, CD4, low CD3, low CD2 and negative for CD7, CD38, HLA-DR and CD8. The trephine biopsy showed a 7% infiltration of SS cells. The CD4:CD8 ratio in peripheral blood (pb) was massively increased at 76.67, with 63.5% of white blood cells expressing a SS immune phenotype. The conditioning regimen included 30 mg/m2 fludarabine on days -5, -4 and -3 and total body irradiation with 2 Gy on day -1. Immunosuppression consisted of cyclosporine A from day-1 and mycophenolate mofetil from day 0. The patient received 6.55x106 CD34+ cells and 1.11x108 CD3+ cells/kg body weight. Bone marrow evaluation on day 28 still showed persistent SS cells by flow cytometry. After tapering immunosuppression until day 169, the CD4:CD8 ratio in pb normalized. CR was documented on day 169 after alloHSCT and is now ongoing for almost 3 years after alloHSCT. Conclusions: We confirm that an alloHSCT can be a curative option for refractory patients with SS. The achievement of a CR after tapering the immunosuppressive therapy indicates a significant role of the GvL effect. In present treatment algorithms for patients with SS, the timing of an alloHSCT and the intensity of conditioning should be further explored.
Keywords: Sezary syndrome; allogeneic hematopietic stem cell transplantation; brentuximab vedodin; graft vs leukemia effect; non-myeloablative conditioning.
Copyright © 2021 Franke, Dumann, Jentzsch, Monecke, Doehring, Nehring-Vucinic, Schwind, Niederwieser, Platzbecker, Ziemer and Vucinic.
Conflict of interest statement
G-NF, MJ, SS, UP, DN, KD, MZ, and VV receive honoraria from Takeda. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- Agar NS, Wedgeworth E, Crichton S, Mitchell TJ, Cox M, Ferreira S, et al. Survival Outcomes and Prognostic Factors in Mycosis Fungoides/Sézary Syndrome: Validation of the Revised International Society for Cutaneous Lymphomas/European Organisation for Research and Treatment of Cancer Staging Proposal. J Clin Oncol (2010) 28:4730–9. doi: 10.1200/JCO.2009.27.7665 - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials

