The Role of Immune Checkpoint Blockade in the Hepatocellular Carcinoma: A Review of Clinical Trials
- PMID: 34956912
- PMCID: PMC8692256
- DOI: 10.3389/fonc.2021.801379
The Role of Immune Checkpoint Blockade in the Hepatocellular Carcinoma: A Review of Clinical Trials
Abstract
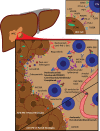
The prevalence of primary liver cancer is rapidly rising all around the world. Hepatocellular carcinoma (HCC) is the most common type of primary liver cancer. Unfortunately, the traditional treatment methods to cure HCC showed poor efficacy in patients who are not candidates for liver transplantation. Until recently, tyrosine kinase inhibitors (TKIs) were the front-line treatment for unresectable liver cancer. However, rapidly emerging new data has drastically changed the landscape of HCC treatment. The combination treatment of atezolizumab plus bevacizumab (immunotherapy plus anti-VEGF) was shown to provide superior outcomes and has become the new standard first-line treatment for unresectable or metastatic HCC. Currently, ongoing clinical trials with immune checkpoint blockade (ICB) have focused on assessing the benefit of antibodies against programmed cell death 1 (PD-1), programmed cell death-ligand 1 (PD-L1), and cytotoxic T-lymphocyte- associated antigen 4 (CTLA-4) as monotherapies or combination therapies in patients with HCC. In this review, we briefly discuss the mechanisms underlying various novel immune checkpoint blockade therapies and combination modalities along with recent/ongoing clinical trials which may generate innovative new treatment approaches with potential new FDA approvals for HCC treatment in the near future.
Keywords: clinical trials; hepatocellular carcinoma (HCC); immune checkpoint blockade; immunotherapy; liver cancer (LC).
Copyright © 2021 Ozer, George, Goksu, George and Sahin.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
New insights into checkpoint inhibitor immunotherapy and its combined therapies in hepatocellular carcinoma: from mechanisms to clinical trials.Int J Biol Sci. 2022 Mar 28;18(7):2775-2794. doi: 10.7150/ijbs.70691. eCollection 2022. Int J Biol Sci. 2022. PMID: 35541908 Free PMC article. Review.
-
Strategies to Improve the Antitumor Effect of Immunotherapy for Hepatocellular Carcinoma.Front Immunol. 2021 Nov 26;12:783236. doi: 10.3389/fimmu.2021.783236. eCollection 2021. Front Immunol. 2021. PMID: 34899747 Free PMC article. Review.
-
Immunotherapy and chimeric antigen receptor T-cell therapy in hepatocellular carcinoma.Chin Clin Oncol. 2021 Feb;10(1):11. doi: 10.21037/cco-20-231. Chin Clin Oncol. 2021. PMID: 33541088 Review.
-
Current landscape and future of dual anti-CTLA4 and PD-1/PD-L1 blockade immunotherapy in cancer; lessons learned from clinical trials with melanoma and non-small cell lung cancer (NSCLC).J Immunother Cancer. 2018 May 16;6(1):39. doi: 10.1186/s40425-018-0349-3. J Immunother Cancer. 2018. PMID: 29769148 Free PMC article. Review.
-
An update on atezolizumab for hepatocellular carcinoma.Drugs Today (Barc). 2021 Jun;57(6):365-375. doi: 10.1358/dot.2021.57.6.3264116. Drugs Today (Barc). 2021. PMID: 34151903
Cited by
-
CTLA-4 silencing could promote anti-tumor effects in hepatocellular.Med Oncol. 2024 Jul 2;41(8):193. doi: 10.1007/s12032-024-02361-1. Med Oncol. 2024. PMID: 38955918
-
Current Landscape of Immune Checkpoint Inhibitor Therapy for Hepatocellular Carcinoma.Cancers (Basel). 2022 Apr 16;14(8):2018. doi: 10.3390/cancers14082018. Cancers (Basel). 2022. PMID: 35454923 Free PMC article. Review.
-
Proof of concept nanotechnological approach to in vitro targeting of malignant melanoma for enhanced immune checkpoint inhibition.Sci Rep. 2023 May 8;13(1):7462. doi: 10.1038/s41598-023-34638-2. Sci Rep. 2023. PMID: 37156818 Free PMC article.
-
A Paradigm Shift in Primary Liver Cancer Therapy Utilizing Genomics, Molecular Biomarkers, and Artificial Intelligence.Cancers (Basel). 2023 May 17;15(10):2791. doi: 10.3390/cancers15102791. Cancers (Basel). 2023. PMID: 37345129 Free PMC article. Review.
-
Response of Scalp and Skull Metastasis to Anti-PD-1 Antibody Combined with Regorafenib Treatment in a Sorafenib-Resistant Hepatocellular Carcinoma Patient and a Literature Review.Onco Targets Ther. 2022 Jun 29;15:703-716. doi: 10.2147/OTT.S365652. eCollection 2022. Onco Targets Ther. 2022. PMID: 35791424 Free PMC article.
References
-
- Bruix J, Qin S, Merle P, Granito A, Huang Y-H, Bodoky G, et al. . Regorafenib for Patients With Hepatocellular Carcinoma Who Progressed on Sorafenib Treatment (RESORCE): A Randomised, Double-Blind, Placebo-Controlled, Phase 3 Trial. Lancet (2017) 389(10064):56–66. doi: 10.1016/S0140-6736(16)32453-9 - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials

