Umbilical Cord Procalcitonin to Detect Early-Onset Sepsis in Newborns: A Promising Biomarker
- PMID: 34956986
- PMCID: PMC8704118
- DOI: 10.3389/fped.2021.779663
Umbilical Cord Procalcitonin to Detect Early-Onset Sepsis in Newborns: A Promising Biomarker
Abstract
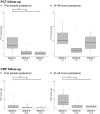
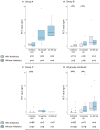
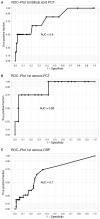
Background: Up to 7% of neonates born in high-income countries receive antibiotics for suspected early-onset sepsis (EOS). Culture-proven neonatal sepsis has a prevalence of 0.2%, suggesting considerable overtreatment. We studied the diagnostic accuracy of umbilical cord blood and infant blood procalcitonin (PCT) in diagnosing EOS to improve antibiotic stewardship. Methods: Umbilical cord blood PCT was tested in newborns ≥ 32 weeks of gestation. Groups were defined as following: A) culture-proven or probable EOS (n = 25); B) Possible EOS, based on risk factors for which antibiotics were administered for <72 h (n = 49); C) Risk factor(s) for EOS without need for antibiotic treatment (n = 181); D) Healthy controls (n = 74). Additionally, venous or capillary blood PCT and C-reactive protein (CRP) were tested if blood drawing was necessary for standard care. Results: Between June 2019 and March 2021, 329 newborns were included. Umbilical cord blood PCT was significantly higher in group A than in group C and D. No difference between venous or arterial samples was found. Sensitivity and specificity for cord blood procalcitonin were 83 and 62%, respectively (cut-off 0.1 ng/mL). Antepartum maternal antibiotic administration was associated with decreased PCT levels in both cord blood and infant blood directly postpartum in all groups combined. Conclusion: Umbilical cord blood PCT levels are increased in newborns ≥32 weeks with a proven or probable EOS and low in newborns with risk factors for infection, but PCT seems not a reliable marker after maternal antibiotic treatment. PCT could be useful to distinguish infected from healthy newborns with or without EOS risk factors.
Keywords: antibiotic stewardship; early-onset sepsis; neonatal infection; procalcitonin; umbilical cord blood.
Copyright © 2021 Dongen, van Leeuwen, de Groot, Vollebregt, Schiering, Wevers, Euser and van Houten.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
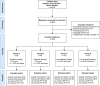
Figures

References
-
- Fleischmann-Struzek C, Goldfarb, DM, Schlattmann, P, Schlapbach, LJ, Reinhart, K, Kissoon, N,. The Global Burden of Paediatric Neonatal Sepsis: A Systematic Review. (2018) Available online at: www.thelancet.com/respiratory (accessed January 14, 2021). - PubMed
-
- Hasperhoven G, Al-Nasiry S, Bekker V, Villamor E, Kramer B. Universal screening versus risk-based protocols for antibiotic prophylaxis during childbirth to prevent early-onset group B streptococcal disease: a systematic review and meta-analysis. BJOG. (2020) 127:680–91. 10.1111/1471-0528.16085 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

