APOE4 derived from astrocytes leads to blood-brain barrier impairment
- PMID: 34957486
- PMCID: PMC9586546
- DOI: 10.1093/brain/awab478
APOE4 derived from astrocytes leads to blood-brain barrier impairment
Abstract
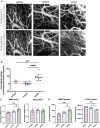
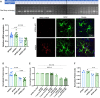
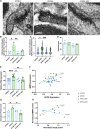
Apolipoprotein E (ApoE) is a multifaceted secreted molecule synthesized in the CNS by astrocytes and microglia, and in the periphery largely by the liver. ApoE has been shown to impact the integrity of the blood-brain barrier, and, in humans, the APOE4 allele of the gene is reported to lead to a leaky blood-brain barrier. We used allele specific knock-in mice expressing each of the common (human) ApoE alleles, and longitudinal multiphoton intravital microscopy, to directly monitor the impact of various ApoE isoforms on blood-brain barrier integrity. We found that humanized APOE4, but not APOE2 or APOE3, mice show a leaky blood-brain barrier, increased MMP9, impaired tight junctions, and reduced astrocyte end-foot coverage of blood vessels. Removal of astrocyte-produced ApoE4 led to the amelioration of all phenotypes while the removal of astrocyte-produced ApoE3 had no effect on blood-brain barrier integrity. This work shows a cell specific gain of function effect of ApoE4 in the dysfunction of the BBB and implicates astrocyte production of ApoE4, possibly as a function of astrocytic end foot interactions with vessels, as a key regulator of the integrity of the blood-brain barrier.
Keywords: Alzheimer's disease; apolipoprotein E; astrocytes; blood–brain barrier.
© The Author(s) 2022. Published by Oxford University Press on behalf of the Guarantors of Brain.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

