Long-term cannabidiol treatment for seizures in patients with tuberous sclerosis complex: An open-label extension trial
- PMID: 34957550
- PMCID: PMC9305454
- DOI: 10.1111/epi.17150
Long-term cannabidiol treatment for seizures in patients with tuberous sclerosis complex: An open-label extension trial
Abstract
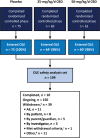
Objective: To evaluate the long-term safety and efficacy of add-on cannabidiol (CBD) in patients with seizures associated with tuberous sclerosis complex (TSC) in the open-label extension (OLE) of the randomized, placebo-controlled phase 3 trial GWPCARE6 (NCT02544763). Results of an interim (February 2019 data cut) analysis are reported.
Methods: Patients who completed the randomized trial enrolled to receive CBD (Epidiolex® in the United States; Epidyolex® in the EU; 100 mg/mL oral solution). The initial target dose was 25 mg/kg/day, which, based on response and tolerability, could be decreased or increased up to 50 mg/kg/day. The primary end point was safety. Key secondary end points included percentage reduction in TSC-associated (countable focal and generalized) seizures, responder rates, and Subject/Caregiver Global Impression of Change (S/CGIC).
Results: Of 201 patients who completed the randomized phase, 199 (99%) entered the OLE. Mean age was 13 years (range, 1-57). At the time of analysis, 5% of patients had completed treatment, 20% had withdrawn, and 75% were ongoing. One-year retention rate was 79%. Median treatment time was 267 days (range, 18-910) at a 27 mg/kg/day mean modal dose. Most patients (92%) had an adverse event (AE). Most common AEs were diarrhea (42%), seizure (22%), and decreased appetite (20%). AEs led to permanent discontinuation in 6% of patients. There was one death that was deemed treatment unrelated by the investigator. Elevated liver transaminases occurred in 17 patients (9%) patients; 12 were taking valproate. Median percentage reductions in seizure frequency (12-week windows across 48 weeks) were 54%-68%. Seizure responder rates (≥50%, ≥75%, 100% reduction) were 53%-61%, 29%-45%, and 6%-11% across 12-week windows for 48 weeks. Improvement on the S/CGIC scale was reported by 87% of patients/caregivers at 26 weeks.
Significance: In patients with TSC, long-term add-on CBD treatment was well tolerated and sustainably reduced seizures through 48 weeks, with most patients/caregivers reporting global improvement.
Keywords: antiseizure medication; cannabidiol; epilepsy; focal seizures; treatment-resistant epilepsy; tuberous sclerosis complex.
© 2021 The Authors. Epilepsia published by Wiley Periodicals LLC on behalf of International League Against Epilepsy.
Conflict of interest statement
Elizabeth A. Thiele received support from GW Research Ltd as an investigator during the conduct of the trial; outside of the current work, she serves as a principal investigator on clinical trials for GW Research Ltd and Zogenix and serves as a consultant for Aquestive Therapeutics, Biocodex, West Therapeutics, Greenwich Biosciences, and Zogenix. E. Martina Bebin received support from GW Research Ltd as a site principal investigator during the conduct of the trial and serves as a consultant for Greenwich Biosciences and Biocodex. Francis Filloux and Patrick Kwan have nothing to disclose. Rachael Loftus is a full‐time employee of GW Research Ltd. Farhad Sahebkar is a full‐time employee of Greenwich Biosciences. Steven Sparagana has received personal compensation for serving as a consultant for Greenwich Biosciences and Nobelpharma. His institution has received research support from Greenwich Biosciences, Tuberous Sclerosis Complex Alliance, and Novartis. He has a noncompensated relationship as a professional advisory board member or committee member with Tuberous Sclerosis Complex Alliance. James Wheless has received personal compensation for serving as a consultant for Eisai, Supernus, Aquestive, GW Pharmaceuticals companies, and Neurelis. He has served on a speakers bureau for LivaNova, Eisai, Supernus, GW Pharmaceuticals companies, UCB, BioMarin, and Zogenix. We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
Figures



References
-
- Curatolo P, Bombardieri R, Jozwiak S. Tuberous sclerosis. Lancet. 2008;372:657–68. - PubMed
-
- Crino PB, Nathanson KL, Henske EP. The tuberous sclerosis complex. N Engl J Med. 2006;355:1345–56. - PubMed
-
- Chan JA, Zhang H, Roberts PS, Jozwiak S, Wieslawa G, Lewin‐Kowalik J, et al. Pathogenesis of tuberous sclerosis subependymal giant cell astrocytomas: biallelic inactivation of TSC1 or TSC2 leads to mTOR activation. J Neuropathol Exp Neurol. 2004;63:1236–42. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

