Strain-Promoted Azide-Alkyne Cycloaddition-Based PSMA-Targeting Ligands for Multimodal Intraoperative Tumor Detection of Prostate Cancer
- PMID: 34957825
- PMCID: PMC8778659
- DOI: 10.1021/acs.bioconjchem.1c00537
Strain-Promoted Azide-Alkyne Cycloaddition-Based PSMA-Targeting Ligands for Multimodal Intraoperative Tumor Detection of Prostate Cancer
Abstract
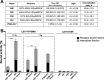
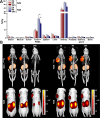
Strain-promoted azide-alkyne cycloaddition (SPAAC) is a straightforward and multipurpose conjugation strategy. The use of SPAAC to link different functional elements to prostate-specific membrane antigen (PSMA) ligands would facilitate the development of a modular platform for PSMA-targeted imaging and therapy of prostate cancer (PCa). As a first proof of concept for the SPAAC chemistry platform, we synthesized and characterized four dual-labeled PSMA ligands for intraoperative radiodetection and fluorescence imaging of PCa. Ligands were synthesized using solid-phase chemistry and contained a chelator for 111In or 99mTc labeling. The fluorophore IRDye800CW was conjugated using SPAAC chemistry or conventional N-hydroxysuccinimide (NHS)-ester coupling. Log D values were measured and PSMA specificity of these ligands was determined in LS174T-PSMA cells. Tumor targeting was evaluated in BALB/c nude mice with subcutaneous LS174T-PSMA and LS174T wild-type tumors using μSPECT/CT imaging, fluorescence imaging, and biodistribution studies. SPAAC chemistry increased the lipophilicity of the ligands (log D range: -2.4 to -4.4). In vivo, SPAAC chemistry ligands showed high and specific accumulation in s.c. LS174T-PSMA tumors up to 24 h after injection, enabling clear visualization using μSPECT/CT and fluorescence imaging. Overall, no significant differences between the SPAAC chemistry ligands and their NHS-based counterparts were found (2 h p.i., p > 0.05), while 111In-labeled ligands outperformed the 99mTc ligands. Here, we demonstrate that our newly developed SPAAC-based PSMA ligands show high PSMA-specific tumor targeting. The use of click chemistry in PSMA ligand development opens up the opportunity for fast, efficient, and versatile conjugations of multiple imaging moieties and/or drugs.
Conflict of interest statement
The authors declare the following competing financial interest(s): Y. Derks, D. Lwik, S. Heskamp, P. Laverman and M. Rijpkema are applicants of patent: EP21155853 - PSMA-targeting ligands for multimodal applications. No other potential conflicts of interest relevant to this article exist.
Figures




References
-
- Lütje S.; Heskamp S.; Franssen G. M.; Frielink C.; Kip A.; Hekman M.; Fracasso G.; Colombatti M.; Herrmann K.; Boerman O. C.; et al. Development and characterization of a theranostic multimodal anti-PSMA targeting agent for imaging, surgical guidance, and targeted photodynamic therapy of PSMA-expressing tumors. Theranostics 2019, 9, 2924–2938. 10.7150/thno.35274. - DOI - PMC - PubMed
-
- Lütje S.; Rijpkema M.; Franssen G. M.; Fracasso G.; Helfrich W.; Eek A.; Oyen W. J.; Colombatti M.; Boerman O. C. Dual-Modality Image-Guided Surgery of Prostate Cancer with a Radiolabeled Fluorescent Anti-PSMA Monoclonal Antibody. J. Nucl. Med. 2014, 55, 995–1001. 10.2967/jnumed.114.138180. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

