Duodenal mucosal resurfacing with a GLP-1 receptor agonist increases postprandial unconjugated bile acids in patients with insulin-dependent type 2 diabetes
- PMID: 34957857
- PMCID: PMC8858668
- DOI: 10.1152/ajpendo.00337.2021
Duodenal mucosal resurfacing with a GLP-1 receptor agonist increases postprandial unconjugated bile acids in patients with insulin-dependent type 2 diabetes
Abstract
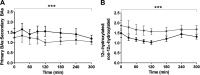
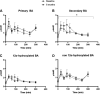
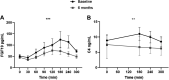
Duodenal mucosal resurfacing (DMR) is a new endoscopic ablation technique aimed at improving glycemia and metabolic control in patients with type 2 diabetes mellitus (T2DM). DMR appears to improve insulin resistance, which is the root cause of T2DM, but its mechanism of action is largely unknown. Bile acids function as intestinal signaling molecules in glucose and energy metabolism via the activation of farnesoid X receptor and secondary signaling [e.g., via fibroblast growth factor 19 (FGF19)], and are linked to metabolic health. We investigated the effect of DMR and glucagon-like peptide-1 (GLP-1) on postprandial bile acid responses in 16 patients with insulin-dependent T2DM, using mixed meal tests performed at the baseline and 6 mo after the DMR procedure. The combination treatment allowed discontinuation of insulin treatment in 11/16 (69%) of patients while improving glycemic and metabolic health. We found increased postprandial unconjugated bile acid responses (all P < 0.05), an overall increased secondary bile acid response (P = 0.036) and a higher 12α-hydroxylated:non-12α-hydroxylated ratio (P < 0.001). Total bile acid concentrations were unaffected by the intervention. Postprandial FGF19 and 7-α-hydroxy-4-cholesten-3-one (C4) concentrations decreased postintervention (both P < 0.01). Our study demonstrates that DMR with GLP-1 modulates the postprandial bile acid response. The alterations in postprandial bile acid responses may be the result of changes in the microbiome, ileal bile acid uptake and improved insulin sensitivity. Controlled studies are needed to elucidate the mechanism linking the combination treatment to metabolic health and bile acids.NEW & NOTEWORTHY Glycemic and metabolic improvements are seen in patients with type 2 diabetes after replacing their insulin therapy with DMR and GLP-1. These changes are accompanied by changes in postprandial bile acid concentrations: increased unconjugated and secondary bile acids.
Keywords: DMR; bile acids; diabetes type 2; duodenal ablation; duodenal mucosal resurfacing.
Conflict of interest statement
J.J.G.H.M.B. received research support from Fractyl Laboratories Inc. for IRB-based studies and received a consultancy fee for a single advisory board meeting of Fractyl in September 2019. None of the other authors has any conflicts of interest, financial or otherwise, to disclose.
Figures


References
-
- Davies MJ, D’Alessio DA, Fradkin J, Kernan WN, Mathieu C, Mingrone G, Rossing P, Tsapas A, Wexler DJ, Buse JB. Management of hyperglycemia in type 2 diabetes, 2018. A Consensus Report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 41: 2669–2701, 2018. doi:10.2337/dci18-0033. - DOI - PMC - PubMed
-
- Adams TD, Davidson LE, Litwin SE, Kolotkin RL, LaMonte MJ, Pendleton RC, Strong MB, Vinik R, Wanner NA, Hopkins PN, Gress RE, Walker JM, Cloward TV, Nuttall RT, Hammoud A, Greenwood JL, Crosby RD, McKinlay R, Simper SC, Smith SC, Hunt SC. Health benefits of gastric bypass surgery after 6 years. JAMA 308: 1122–1131, 2012. doi:10.1001/2012.jama.11164. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

