Superior even skin tone and anti-ageing benefit of a combination of 4-hexylresorcinol and niacinamide
- PMID: 34958693
- PMCID: PMC9305876
- DOI: 10.1111/ics.12759
Superior even skin tone and anti-ageing benefit of a combination of 4-hexylresorcinol and niacinamide
Abstract
Objectives: To demonstrate the synergistic effect of 4-hexylresorcinol (4-HR) with niacinamide in boosting anti-melanogenic efficacy in vitro and establish the in vivo efficacy and safety of the combination in a human trial.
Methods: Primary human epidermal melanocytes and 3D pigmented skin equivalents were treated with 4-HR, niacinamide, and their combinations for their effect on pigmentation. This was followed by a randomized, double-blind, split-face clinical study in Chinese subjects, and effects on skin tone, hyperpigmentation, fine lines and wrinkles, hydration, and skin firmness were measured for a 12-week study period.
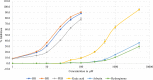
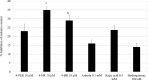
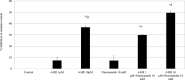
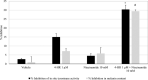
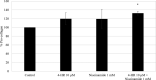
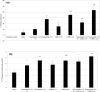
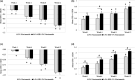
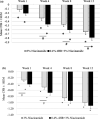
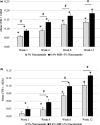
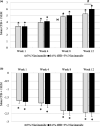
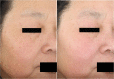
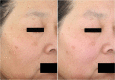
Results: In vitro tyrosinase enzyme activity studies showed that 4-HR is one of the most potent tyrosinase inhibitors. The combination of 4-HR and niacinamide showed a synergistic reduction in melanin production in cultured melanocytes and lightened the 3D skin equivalent model. In vitro as well as in the human trial, the combination of 4-HR and niacinamide showed significantly improved efficacy over niacinamide alone on hyperpigmentation spots as measured by L*, the visual appearance of fine lines and wrinkles in crow's feet and perioral area and skin firmness, with no product-related adverse events.
Conclusions: A formulation containing a combination of 4-HR and niacinamide delivered superior skin tone and anti-ageing benefits significantly better than niacinamide alone with no adverse events. This study demonstrates that a product designed to affect multiple pathways of melanogenesis, inflammation, and ageing may provide an additional treatment option, beyond hydroquinone and retinoids, for hyperpigmentation and ageing.
Objectifs: Démontrer l’effet synergique du 4-hexylrésorcinol (4-HR) associé au niacinamide pour stimuler in vitro l’efficacité antimélanogène, et établir l’efficacité et la sécurité d’emploi in vivo de cette association dans un essai chez l’homme. MÉTHODES: Des mélanocytes épidermiques humains primaires et des équivalents cutanés pigmentés en 3D ont été traités avec du 4-HR, du niacinamide et leurs combinaisons pour leur effet sur la pigmentation. Ceci a été suivi d’une étude clinique randomisée, en double aveugle en hémi-visage chez des sujets chinois, et les effets sur le teint, l’hyperpigmentation, les rides et ridules, l’hydratation et la fermeté de la peau ont été mesurés pendant une durée d’étude de 12 semaines. RÉSULTATS: Les études in vitro sur l’activité enzymatique de la tyrosinase ont montré que le 4-HR est l’un des inhibiteurs de la tyrosinase les plus puissants. L’association du 4-HR et du niacinamide a montré une réduction synergique de la production de mélanine dans les mélanocytes de culture et donné de la luminosité au modèle cutané 3D équivalent. Également in vitro avec l’étude chez l’homme, l’association du 4-HR et du niacinamide a fait ressortir une efficacité significativement plus élevée qu’avec le niacinamide seul sur les taches d’hyperpigmentation mesurées par L*, l’aspect visuel des rides et ridules des pattes d’oie et de la zone périorale, et la fermeté de la peau, sans événements indésirables liés au produit.
Conclusions: Une formulation contenant une association de 4-HR et de niacinamide a permis d’obtenir un teint et un effet anti-âge nettement supérieurs à ceux du niacinamide seul, sans événements indésirables. Cette étude démontre qu’un produit conçu pour toucher plusieurs voies de mélanogenèse, d’inflammation et de vieillissement peut constituer une nouvelle option thérapeutique, au-delà de l’hydroquinone et des rétinoïdes, pour l’hyperpigmentation et le vieillissement.
Keywords: cell culture; human volunteer trial; niacinamide; resorcinols; skin physiology/structure; spectroscopy.
© 2022 Unilever R&D. International Journal of Cosmetic Science published by John Wiley & Sons Ltd on behalf of Society of Cosmetic Scientists and Societe Francaise de Cosmetologie.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Greatens A, Hakozaki T, Koshoffer A, Epstein H, Schwemberger S, Babcock G et al. Effective inhibition of melanosome transfer to keratinocytes by lectins and niacinamide is reversible. Exp Dermatol. 2005;14(7):498–508. - PubMed
-
- Hakozaki T, Minwalla L, Zhuang J, Chhoa M, Matsubara A, Miyamoto K, Greatens A, Hillebrand GG, Bissett DL, Boissy RE. The effect of niacinamide on reducing cutaneous pigmentation and suppression of melanosome transfer. Br J Dermatol. 2002;147(1):20–31. - PubMed
-
- Chen AC, Damian DL. Nicotinamide and the skin. Australas J Dermatol. 2014;55(3):169–75. - PubMed
-
- Makino ET, Mehta RC, Banga A, Jain P, Sigler ML, Sonti S. Evaluation of a hydroquinone‐free skin brightening product using in‐vitro inhibition of melanogenesis and clinical reduction of ultraviolet‐induced hyperpigmentation. J Drugs Dermatol. 2013;12(3):S16–20. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

