Transdermal Drug Delivery in the Pig Skin
- PMID: 34959299
- PMCID: PMC8707795
- DOI: 10.3390/pharmaceutics13122016
Transdermal Drug Delivery in the Pig Skin
Abstract
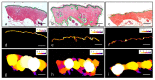
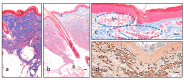
Transdermal delivery can be accomplished through various mechanisms including formulation optimization, epidermal stratum corneum barrier disruption, or directly by removing the stratum corneum layer. Microneedling, electroporation, a combination of both and also the intradermal injection known as mesotherapy have proved efficacy in epidermal-barrier disruption. Here we analyzed the effects of these methods of epidermal-barrier disruption in the structure of the skin and the absorption of four compounds with different characteristics and properties (ketoprofen, biotin, caffein, and procaine). Swine skin (Pietrain x Durox) was used as a human analogue, both having similar structure and pharmacological release. They were biopsied at different intervals, up to 2 weeks after application. High-pressure liquid chromatography and brightfield microscopy were performed, conducting a biometric analysis and measuring histological structure and vascular status. The performed experiments led to different results in the function of the studied molecules: ketoprofen and biotin had the best concentrations with intradermal injections, while delivery methods for obtaining procaine and caffein maximum concentrations changed on the basis of the lapsed time. The studied techniques did not produce significant histological alterations after their application, except for an observed increase in Langerhans cells and melanocytes after applying electroporation, and an epidermal thinning after using microneedles, with variable results regarding dermal thickness. Although all the studied barrier disruptors can accomplish transdermal delivery, the best disruptor is dependent on the particular molecule.
Keywords: electroporation; intradermal injection; mesotherapy; microneedling; morphology; skin; transdermal delivery.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






References
Grants and funding
LinkOut - more resources
Full Text Sources

