Investigating Structural Property Relationships to Enable Repurposing of Pharmaceuticals as Zinc Ionophores
- PMID: 34959313
- PMCID: PMC8704213
- DOI: 10.3390/pharmaceutics13122032
Investigating Structural Property Relationships to Enable Repurposing of Pharmaceuticals as Zinc Ionophores
Abstract
The importance of zinc in biology has gained greater recognition in recent years due to its essential contributions to the function of many endogenous enzymes. Disruption of zinc homeostasis may be useful in treating pathological conditions, such as Alzheimer's, and for antiviral purposes. Despite the growth of knowledge and increased interest in zinc, little is known about the structure and function of zinc ionophores. In this study we analyse the Cambridge Structural Database and solution complexation studies found in the literature to identify key functional groups which may confer zinc ionophorism. Pharmaceuticals, nutraceuticals and amino acids with these functionalities were selected to enable us to explore the translatability of ionophoric activity from in vitro assays to cellular systems. We find that although certain species may complex to zinc in the solid and solution states, and may carry ions across simple membrane systems, this does not necessarily translate into ionophoric activity. We propose that the CSD can help refine key functionalities but that ionophoric activity must be confirmed in cellular systems.
Keywords: CSD analysis; drug repurposing; ionophore; ionophorism; zinc.
Conflict of interest statement
One of the authorship team (S.R.) is currently an employee of Janssen Pharmaceuticals. There are no other conflicts to declare.
Figures


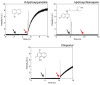
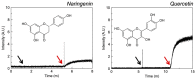



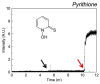
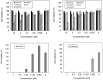






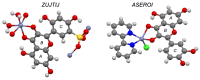
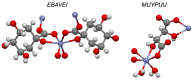
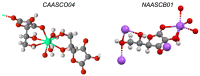



References
-
- Frederickson C.J., Koh J.Y., Bush A.I. The neurobiology of zinc in health and disease. [(accessed on 9 September 2021)];Nat. Rev. Neurosci. 2005 6:449–462. doi: 10.1038/nrn1671. Available online: www.nature.com/reviews/neuro. - DOI - PubMed
-
- Read S.A., Obeid S., Ahlenstiel C., Ahlenstiel G. The Role of Zinc in Antiviral Immunity. [(accessed on 10 September 2021)];Adv. Nutr. 2019 10:696–710. doi: 10.1093/advances/nmz013. Available online: https://pmc/articles/PMC6628855/?report=abstract. - DOI - PMC - PubMed
-
- Frederickson C.J., Maret W., Cuajungco M.P. Zinc and Excitotoxic Brain Injury: A New Model [Internet] [(accessed on 9 September 2021)];Neuroscientist. 2004 10:18–25. doi: 10.1177/1073858403255840. Available online: https://pubmed.ncbi.nlm.nih.gov/14987444/ - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

