Comparative Diagnostic Performance of a Novel Reverse Transcription Loop-Mediated Isothermal Amplification (RT-LAMP) Kit for the Rapid Detection of SARS-CoV-2
- PMID: 34959584
- PMCID: PMC8706056
- DOI: 10.3390/pathogens10121629
Comparative Diagnostic Performance of a Novel Reverse Transcription Loop-Mediated Isothermal Amplification (RT-LAMP) Kit for the Rapid Detection of SARS-CoV-2
Abstract
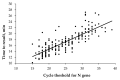
Although the reverse transcription-polymerase chain reaction (RT-PCR) is considered a standard-of-care assay for the laboratory diagnosis of SARS-CoV-2, several limitations of this method have been described. Reverse transcription loop-mediated isothermal amplification (RT-LAMP) is an alternative molecular assay and is potentially able to overcome some intrinsic shortcomings of RT-PCR. In this study, we evaluated the diagnostic performance of the novel HG COVID-19 RT-LAMP assay. In this retrospective analysis, a total of 400 routinely collected leftover nasopharyngeal samples with a known RT-PCR result were tested by means of the HG COVID-19 RT-LAMP assay. The overall sensitivity and specificity values of HG COVID-19 RT-LAMP versus RT-PCR were 97.0% (95% CI: 93.6-98.9%) and 98.5% (95% CI: 95.7-99.7%), respectively. Inter-assay agreement was almost perfect (κ = 0.96). Concordance was perfect in samples with high viral loads (cycle threshold < 30). The average time to a positive result on RT-LAMP was 17 min. HG COVID-19 RT-LAMP is a reliable molecular diagnostic kit for detecting SARS-CoV-2, and its performance is comparable to that of RT-PCR. Shorter turnaround times and the possibility of performing molecular diagnostics in the point-of-care setting make it a valuable option for facilities without sophisticated laboratory equipment.
Keywords: COVID-19; RT-LAMP; RT-PCR; SARS-CoV-2; diagnostic accuracy.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
A Multiplex and Colorimetric Reverse Transcription Loop-Mediated Isothermal Amplification Assay for Sensitive and Rapid Detection of Novel SARS-CoV-2.Front Cell Infect Microbiol. 2021 Jun 29;11:653616. doi: 10.3389/fcimb.2021.653616. eCollection 2021. Front Cell Infect Microbiol. 2021. PMID: 34268131 Free PMC article.
-
Development and Clinical Application of a Rapid and Sensitive Loop-Mediated Isothermal Amplification Test for SARS-CoV-2 Infection.mSphere. 2020 Aug 26;5(4):e00808-20. doi: 10.1128/mSphere.00808-20. mSphere. 2020. PMID: 32848011 Free PMC article.
-
Comparison of COVID-19 laboratory diagnosis by commercial kits: Effectivity of RT-PCR to the RT-LAMP.J Med Virol. 2022 May;94(5):1998-2007. doi: 10.1002/jmv.27559. Epub 2022 Jan 21. J Med Virol. 2022. PMID: 34997587 Free PMC article.
-
Reverse Transcriptase Loop Mediated Isothermal Amplification (RT-LAMP) for COVID-19 diagnosis: a systematic review and meta-analysis.Pathog Glob Health. 2021 Jul;115(5):281-291. doi: 10.1080/20477724.2021.1933335. Epub 2021 Jun 4. Pathog Glob Health. 2021. PMID: 34086539 Free PMC article.
-
Loop-Mediated Isothermal Amplification (LAMP): A Rapid, Sensitive, Specific, and Cost-Effective Point-of-Care Test for Coronaviruses in the Context of COVID-19 Pandemic.Biology (Basel). 2020 Jul 22;9(8):182. doi: 10.3390/biology9080182. Biology (Basel). 2020. PMID: 32707972 Free PMC article. Review.
Cited by
-
A review of current effective COVID-19 testing methods and quality control.Arch Microbiol. 2023 May 17;205(6):239. doi: 10.1007/s00203-023-03579-9. Arch Microbiol. 2023. PMID: 37195393 Free PMC article. Review.
-
Laboratory-based molecular test alternatives to RT-PCR for the diagnosis of SARS-CoV-2 infection.Cochrane Database Syst Rev. 2024 Oct 14;10(10):CD015618. doi: 10.1002/14651858.CD015618. Cochrane Database Syst Rev. 2024. PMID: 39400904
References
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous