β-lactam Resistance in Pseudomonas aeruginosa: Current Status, Future Prospects
- PMID: 34959593
- PMCID: PMC8706265
- DOI: 10.3390/pathogens10121638
β-lactam Resistance in Pseudomonas aeruginosa: Current Status, Future Prospects
Abstract
Pseudomonas aeruginosa is a major opportunistic pathogen, causing a wide range of acute and chronic infections. β-lactam antibiotics including penicillins, carbapenems, monobactams, and cephalosporins play a key role in the treatment of P. aeruginosa infections. However, a significant number of isolates of these bacteria are resistant to β-lactams, complicating treatment of infections and leading to worse outcomes for patients. In this review, we summarize studies demonstrating the health and economic impacts associated with β-lactam-resistant P. aeruginosa. We then describe how β-lactams bind to and inhibit P. aeruginosa penicillin-binding proteins that are required for synthesis and remodelling of peptidoglycan. Resistance to β-lactams is multifactorial and can involve changes to a key target protein, penicillin-binding protein 3, that is essential for cell division; reduced uptake or increased efflux of β-lactams; degradation of β-lactam antibiotics by increased expression or altered substrate specificity of an AmpC β-lactamase, or by the acquisition of β-lactamases through horizontal gene transfer; and changes to biofilm formation and metabolism. The current understanding of these mechanisms is discussed. Lastly, important knowledge gaps are identified, and possible strategies for enhancing the effectiveness of β-lactam antibiotics in treating P. aeruginosa infections are considered.
Keywords: AmpC; PBP3; antibiotic efflux; antibiotic resistance; carbapenem; carbapenemase; cephalosporin; cystic fibrosis; nosocomial infection; β-lactamase.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

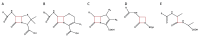
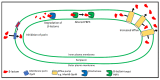
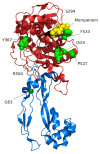


References
-
- Sievert D.M., Ricks P., Edwards J.R., Schneider A., Patel J., Srinivasan A., Kallen A., Limbago B., Fridkin S. Antimicrobial-resistant pathogens associated with healthcare-associated infections summary of data reported to the National Healthcare Safety Network at the Centers for Disease Control and Prevention, 2009–2010. Infect. Control Hosp. Epidemiol. 2013;34:1–14. doi: 10.1086/668770. - DOI - PubMed
-
- Nathwani D., Raman G., Sulham K., Gavaghan M., Menon V. Clinical and economic consequences of hospital-acquired resistant and multidrug-resistant Pseudomonas aeruginosa infections: A systematic review and meta-analysis. Antimicrob. Resist. Infect. Control. 2014;3:32. doi: 10.1186/2047-2994-3-32. - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

