First-In-Human Results on the Biodistribution, Pharmacokinetics, and Dosimetry of [177Lu]Lu-DOTA.SA.FAPi and [177Lu]Lu-DOTAGA.(SA.FAPi)2
- PMID: 34959613
- PMCID: PMC8707268
- DOI: 10.3390/ph14121212
First-In-Human Results on the Biodistribution, Pharmacokinetics, and Dosimetry of [177Lu]Lu-DOTA.SA.FAPi and [177Lu]Lu-DOTAGA.(SA.FAPi)2
Abstract
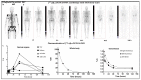
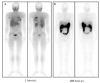
Recently, great interest has been gained regarding fibroblast activation protein (FAP) as an excellent target for theranostics. Several FAP inhibitor molecules such as [68Ga]Ga-labelled FAPI-02, 04, 46, and DOTA.SA.FAPi have been introduced and are highly promising molecular targets from the imaging point of view. FAP inhibitors introduced via bifunctional DOTA and DOTAGA chelators offer the possibility to complex Lutetium-177 due to an additional coordination site, and are suitable for theranostic applications owing to the increased tumor accumulation and prolonged tumor retention time. However, for therapeutic applications, very little has been accomplished, mainly due to residence times of the compounds. In an attempt to develop a promising therapeutic radiopharmaceutical, the present study aimed to evaluate and compare the biodistribution, pharmacokinetics, and dosimetry of [177Lu]Lu-DOTA.SA.FAPi, and [177Lu]Lu-DOTAGA.(SA.FAPi)2 in patients with various cancers. The FAPi agents, [177Lu]Lu-DOTA.SA.FAPi and [177Lu]Lu-DOTAGA.(SA.FAPi)2, were administered in two different groups of patients. Three patients (mean age-50 years) were treated with a median cumulative activity of 2.96 GBq (IQR: 2.2-3 GBq) [177Lu]Lu-DOTA.SA.FAPi and seven (mean age-51 years) were treated with 1.48 GBq (IQR: 0.6-1.5) of [177Lu]Lu-DOTAGA.(SA.FAPi)2. Patients in both the groups underwent serial imaging whole-body planar and SPECT/CT scans that were acquired between 1 h and 168 h post-injection (p.i.). The residence time and absorbed dose estimate in the source organs and tumor were calculated using OLINDA/EXM 2.2 software. Time versus activity graphs were plotted to determine the effective half-life (Te) in the whole body and lesions for both the radiotracers. Physiological uptake of [177Lu]Lu-DOTA.SA.FAPi was observed in the kidneys, colon, pancreas, liver, gall bladder, oral mucosa, lacrimal glands, and urinary bladder contents. Physiological biodistribution of [177Lu]Lu-DOTAGA.(SA.FAPi)2 involved liver, gall bladder, colon, pancreas, kidneys, and urinary bladder contents, lacrimal glands, oral mucosa, and salivary glands. In the [177Lu]Lu-DOTA.SA.FAPi group, the highest absorbed doses were noted in the kidneys (0.618 ± 0.015 Gy/GBq), followed by the colon (right colon: 0.472 Gy/GBq and left colon: 0.430 Gy/GBq). In the [177Lu]Lu-DOTAGA.(SA.FAPi)2 group, the colon received the highest absorbed dose (right colon: 1.160 Gy/GBq and left colon: 2.870 Gy/GBq), and demonstrated a significantly higher mean absorbed dose than [177Lu]Lu-DOTA.SA.FAPi (p < 0.011). [177Lu]Lu-DOTAGA.(SA.FAPi)2 had significantly longer median whole-body Te compared to that of [177Lu]Lu-DOTA.SA.FAPi [46.2 h (IQR: 38.5-70.1) vs. 23.1 h (IQR: 17.8-31.5); p-0.0167]. The Te of tumor lesions was significantly higher for [177Lu]Lu-DOTAGA.(SA.FAPi)2 compared to [177Lu]Lu-DOTA.SA.FAPi [86.6 h (IQR: 34.3-94.6) vs. 14 h (IQR: 12.8-15.5); p-0.0004]. The median absorbed doses to the lesions were 0.603 (IQR: 0.230-1.810) Gy/GBq and 6.70 (IQR: 3.40-49) Gy/GBq dose per cycle in the [177Lu]Lu-DOTA.SA.FAPi, and [177Lu]Lu-DOTAGA.(SA.FAPi)2 groups, respectively. The first clinical dosimetry study demonstrated significantly higher tumor absorbed doses with [177Lu]Lu-DOTAGA.(SA.FAPi)2 compared to [177Lu]Lu-DOTA.SA.FAPi. [177Lu]Lu-DOTAGA.(SA.FAPi)2 is safe and unveiled new frontiers to treat various end-stage cancer patients with a theranostic approach.
Keywords: [177Lu]Lu-DOTA.SA.FAPi; [177Lu]Lu-DOTAGA.(SA.FAPi)2; [68Ga]Ga-DOTA.SA.FAPi PET/CT; absorbed dose estimates; biodistribution; dosimetry; effective half-life; pharmacokinetics.
Conflict of interest statement
All the authors included in this manuscript state no conflict of interest.
Figures


References
-
- Loktev A., Lindner T., Burger E.M., Altmann A., Giesel F., Kratochwil C., Debus J., Marmé F., Jäger D., Mier W., et al. development of fibroblast activation protein-targeted radiotracers with improved tumor retention. J. Nucl. Med. 2019;60:1421–1429. doi: 10.2967/jnumed.118.224469. - DOI - PMC - PubMed
-
- Moon E.S., Elvas F., Vliegen G., De Lombaerde S., Vangestel C., De Bruycker S., Bracke A., Eppard E., Greifenstein L., Klasen B., et al. Targeting fibroblast activation protein (FAP): Next generation PET radiotracers using squaramide coupled bifunctional DOTA and DATA5m (superscript) chelators. EJNMMI Radiopharm. Chem. 2020;5:19. doi: 10.1186/s41181-020-00102-z. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

