Promising Antiviral Activity of Agrimonia pilosa Phytochemicals against Severe Acute Respiratory Syndrome Coronavirus 2 Supported with In Vivo Mice Study
- PMID: 34959713
- PMCID: PMC8709118
- DOI: 10.3390/ph14121313
Promising Antiviral Activity of Agrimonia pilosa Phytochemicals against Severe Acute Respiratory Syndrome Coronavirus 2 Supported with In Vivo Mice Study
Abstract
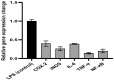
The global emergence of the COVID-19 pandemic caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has focused the entire world's attention toward searching for a potential remedy for this disease. Thus, we investigated the antiviral activity of Agrimonia pilosa ethanol extract (APEE) against SARS-CoV-2 and it exhibited a potent antiviral activity with IC50 of 1.1 ± 0.03 µg/mL. Its mechanism of action was elucidated, and it exhibited a virucidal activity and an inhibition of viral adsorption. Moreover, it presented an immunomodulatory activity as it decreased the upregulation of gene expression of COX-2, iNOS, IL-6, TNF-α, and NF-κB in lipopolysaccharide (LPS)-induced peripheral blood mononuclear cells. A comprehensive analysis of the phytochemical fingerprint of APEE was conducted using LC-ESI-MS/MS technique for the first time. We detected 81 compounds and most of them belong to the flavonoid and coumarin classes. Interestingly, isoflavonoids, procyanidins, and anthocyanins were detected for the first time in A. pilosa. Moreover, the antioxidant activity was evidenced in DPPH (IC50 62.80 µg/mL) and ABTS (201.49 mg Trolox equivalents (TE)/mg) radical scavenging, FRAP (60.84 mg TE/mg), and ORAC (306.54 mg TE/g) assays. Furthermore, the protective effect of APEE was investigated in Lipopolysaccharides (LPS)-induced acute lung injury (ALI) in mice. Lung W/D ratio, serum IL-6, IL-18, IL-1β, HO-1, Caspase-1, caspase-3, TLR-4 expression, TAC, NO, MPO activity, and histopathological examination of lung tissues were assessed. APEE induced a marked downregulation in all inflammation, oxidative stress, apoptosis markers, and TLR-4 expression. In addition, it alleviated all histopathological abnormalities confirming the beneficial effects of APEE in ALI. Therefore, APEE could be a potential source for therapeutic compounds that could be investigated, in future preclinical and clinical trials, in the treatment of patients with COVID-19.
Keywords: ALI; LC-ESI-MS/MS; LPS; SARS-CoV-2; TLR-4; immunomodulatory; plaque reduction.
Conflict of interest statement
The authors declare no conflict of interest.
Figures










References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

