Azacitidine Omega-3 Self-Assemblies: Synthesis, Characterization, and Potent Applications for Myelodysplastic Syndromes
- PMID: 34959720
- PMCID: PMC8706301
- DOI: 10.3390/ph14121317
Azacitidine Omega-3 Self-Assemblies: Synthesis, Characterization, and Potent Applications for Myelodysplastic Syndromes
Abstract
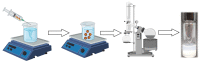
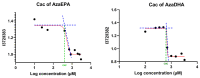
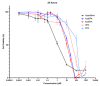
5-Azacitidine, a cytidine analogue used as a hypomethylating agent, is one of the main drugs for the treatment of myelodysplastic syndromes (MDSs) and acute myeloid leukemia (AML) in the elderly. However, after administration, it exhibits several limitations, including restricted diffusion and cellular internalization due to its hydrophilicity, and a rapid enzymatic degradation by adenosine deaminase. The aim of this study was to improve the drug cell diffusion and protect it from metabolic degradation via the synthesis of amphiphilic prodrugs and their potential self-assembly. Azacitidine was conjugated to two different omega-3 fatty acids, eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA). The carboxylic acid group of the omega-3 fatty acids was effectively conjugated to the amine group of the azacitidine base, yielding two amphiphilic prodrugs. Nanoprecipitation of the obtained prodrugs was performed and self-assemblies were successfully obtained for both prodrugs, with a mean diameter of 190 nm, a polydispersity index below 0.2 and a positive zeta potential. The formation of self-assemblies was confirmed using pyrene as a fluorescent dye, and the critical aggregation concentrations were determined: 400 µM for AzaEPA and 688 µM for AzaDHA. Additionally, the stability of the obtained self-assemblies was studied and after 5 days their final stable arrangement was reached. Additionally, cryo-TEM revealed that the self-assemblies attain a multilamellar vesicle supramolecular structure. Moreover, the obtained self-assemblies presented promising cytotoxicity on a leukemia human cell line, having a low IC50 value, comparable to that of free azacitidine.
Keywords: PUFAylation; azacitidine; docosahexaenoic acid; eicosapentaenoic acid; myelodysplastic syndromes; nanomedicine.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Itzykson R., Thépot S., Achour B., Quesnel B., Dreyfus F., Turlure P., Taksin A.-L., Vey N., Koka A.M., De Botton S., et al. Azacytidine (AZA) in MDS (including RAEB-t and CMML) in Patients (pts) ≥ 80 Years: Results of the French ATU Program. Blood. 2009;114:1773. doi: 10.1182/blood.V114.22.1773.1773. - DOI
LinkOut - more resources
Full Text Sources
Research Materials

