Human Milk Oligosaccharides in Cord Blood Are Altered in Gestational Diabetes and Stimulate Feto-Placental Angiogenesis In Vitro
- PMID: 34959807
- PMCID: PMC8705424
- DOI: 10.3390/nu13124257
Human Milk Oligosaccharides in Cord Blood Are Altered in Gestational Diabetes and Stimulate Feto-Placental Angiogenesis In Vitro
Abstract
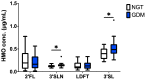
(1) Background: Human milk oligosaccharides (HMOs) are present in maternal serum during pregnancy and their composition is altered in gestational diabetes (GDM). HMOs are also in fetal cord blood and in contact with the feto-placental endothelium, potentially affecting its functions, such as angiogenesis. We hypothesized that cord blood HMOs are changed in GDM and contribute to increased feto-placental angiogenesis, hallmark of GDM. (2) Methods: Using HPLC, we quantified HMOs in cord blood of women with normal glucose tolerance (NGT, n = 25) or GDM (n = 26). We investigated in vitro angiogenesis using primary feto-placental endothelial cells (fpECs) from term placentas after healthy pregnancy (n = 10), in presence or absence of HMOs (100 µg/mL) isolated from human milk, 3'-sialyllactose (3'SL, 30 µg/mL) and lactose (glycan control) and determined network formation (Matrigel assay), proliferation (MTT assays), actin organization (F-actin staining), tube formation (fibrin tube formation assay) and sprouting (spheroid sprouting assay). (3) Results: 3'SL was higher in GDM cord blood. HMOs increased network formation, HMOs and 3'SL increased proliferation and F-actin staining. In fibrin assays, HMOs and 3'SL increased total tube length by 24% and 25% (p < 0.05), in spheroid assays, by 32% (p < 0.05) and 21% (p = 0.056), respectively. Lactose had no effect. (4) Conclusions: Our study suggests a novel role of HMOs in feto-placental angiogenesis and indicates a contribution of HMO composition to altered feto-placental vascularization in GDM.
Keywords: 3′-sialyllactose; angiogenesis; cord blood; fibrin tube formation assay; gestational diabetes mellitus; human milk oligosaccharides; placenta; pregnancy; spheroid sprouting assay; tube formation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Stahl B., Thurl S., Henker J., Siegel M., Finke B., Sawatzki G. Detection of four human milk groups with respect to Lewis-blood-group-dependent oligosaccharides by serologic and chromatographic analysis. Adv. Exp. Med. Biol. 2001;501:299–306. - PubMed
-
- Mank M., Hauner H., Heck A.J.R., Stahl B. Targeted LC-ESI-MS(2) characterization of human milk oligosaccharide diversity at 6 to 16 weeks post-partum reveals clear staging effects and distinctive milk groups. Anal. Bioanal. Chem. 2020;412:6887–6907. doi: 10.1007/s00216-020-02819-x. - DOI - PMC - PubMed
-
- Kunz C., Meyer C., Collado M.C., Geiger L., Garcia-Mantrana I., Bertua-Rios B., Martinez-Costa C., Borsch C., Rudloff S. Influence of Gestational Age, Secretor, and Lewis Blood Group Status on the Oligosaccharide Content of Human Milk. J. Pediatr. Gastroenterol. Nutr. 2017;64:789–798. doi: 10.1097/MPG.0000000000001402. - DOI - PubMed
-
- McGuire M.K., Meehan C.L., McGuire M.A., Williams J.E., Foster J., Sellen D.W., Kamau-Mbuthia E.W., Kamundia E.W., Mbugua S., Moore S.E., et al. What’s normal? Oligosaccharide concentrations and profiles in milk produced by healthy women vary geographically. Am. J. Clin. Nutr. 2017;105:1086–1100. doi: 10.3945/ajcn.116.139980. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

