Investigating the Links between Lower Iron Status in Pregnancy and Respiratory Disease in Offspring Using Murine Models
- PMID: 34960012
- PMCID: PMC8708709
- DOI: 10.3390/nu13124461
Investigating the Links between Lower Iron Status in Pregnancy and Respiratory Disease in Offspring Using Murine Models
Abstract
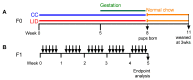
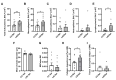
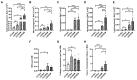
Maternal iron deficiency occurs in 40-50% of all pregnancies and is associated with an increased risk of respiratory disease and asthma in children. We used murine models to examine the effects of lower iron status during pregnancy on lung function, inflammation and structure, as well as its contribution to increased severity of asthma in the offspring. A low iron diet during pregnancy impairs lung function, increases airway inflammation, and alters lung structure in the absence and presence of experimental asthma. A low iron diet during pregnancy further increases these major disease features in offspring with experimental asthma. Importantly, a low iron diet increases neutrophilic inflammation, which is indicative of more severe disease, in asthma. Together, our data demonstrate that lower dietary iron and systemic deficiency during pregnancy can lead to physiological, immunological and anatomical changes in the lungs and airways of offspring that predispose to greater susceptibility to respiratory disease. These findings suggest that correcting iron deficiency in pregnancy using iron supplements may play an important role in preventing or reducing the severity of respiratory disease in offspring. They also highlight the utility of experimental models for understanding how iron status in pregnancy affects disease outcomes in offspring and provide a means for testing the efficacy of different iron supplements for preventing disease.
Keywords: asthma; iron deficiency; offspring; pregnancy; respiratory disease.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Maternal house dust mite exposure during pregnancy enhances severity of house dust mite-induced asthma in murine offspring.J Allergy Clin Immunol. 2017 Nov;140(5):1404-1415.e9. doi: 10.1016/j.jaci.2016.12.972. Epub 2017 Feb 9. J Allergy Clin Immunol. 2017. PMID: 28192144 Free PMC article.
-
Maternal high-fat hypercaloric diet during pregnancy results in persistent metabolic and respiratory abnormalities in offspring.Pediatr Res. 2016 Feb;79(2):278-86. doi: 10.1038/pr.2015.226. Epub 2015 Nov 5. Pediatr Res. 2016. PMID: 26539661 Free PMC article.
-
Prenatal maternal diet affects asthma risk in offspring.J Clin Invest. 2008 Oct;118(10):3265-8. doi: 10.1172/JCI37171. J Clin Invest. 2008. PMID: 18802486 Free PMC article.
-
Effects of maternal-fetal transmission of viruses and other environmental agents on lung development.Pediatr Res. 2020 Jan;87(2):420-426. doi: 10.1038/s41390-019-0657-4. Epub 2019 Nov 7. Pediatr Res. 2020. PMID: 31698410 Free PMC article. Review.
-
Diabetes in pregnancy and lung health in offspring: developmental origins of respiratory disease.Paediatr Respir Rev. 2017 Jan;21:19-26. doi: 10.1016/j.prrv.2016.08.007. Epub 2016 Aug 19. Paediatr Respir Rev. 2017. PMID: 27665512 Review.
Cited by
-
Malnutrition and Allergies: Tipping the Immune Balance towards Health.J Clin Med. 2024 Aug 11;13(16):4713. doi: 10.3390/jcm13164713. J Clin Med. 2024. PMID: 39200855 Free PMC article. Review.
-
Determinants of the Association Between Maternal Anemia and Neonatal Hemoglobin.Nutrients. 2025 Jul 11;17(14):2292. doi: 10.3390/nu17142292. Nutrients. 2025. PMID: 40732917 Free PMC article.
-
Can Stria Gravidarum Predict Surgical Fluid Loss in Cesarean Section?Dermatol Pract Concept. 2023 Jul 1;13(3):e2023175. doi: 10.5826/dpc.1303a175. Dermatol Pract Concept. 2023. PMID: 37557140 Free PMC article.
-
Advances in respiratory physiology in mouse models of experimental asthma.Front Physiol. 2023 Mar 16;14:1099719. doi: 10.3389/fphys.2023.1099719. eCollection 2023. Front Physiol. 2023. PMID: 37008013 Free PMC article. Review.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

