Immunogenicity and Safety of an Intradermal BNT162b2 mRNA Vaccine Booster after Two Doses of Inactivated SARS-CoV-2 Vaccine in Healthy Population
- PMID: 34960122
- PMCID: PMC8703694
- DOI: 10.3390/vaccines9121375
Immunogenicity and Safety of an Intradermal BNT162b2 mRNA Vaccine Booster after Two Doses of Inactivated SARS-CoV-2 Vaccine in Healthy Population
Abstract
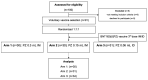
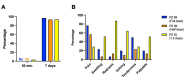
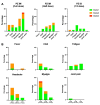
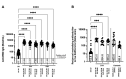
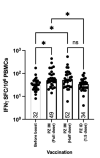
Effective vaccine coverage is urgently needed to tackle the COVID-19 pandemic. Inactivated vaccines have been introduced in many countries for emergency usage, but have only provided limited protection. Heterologous vaccination is a promising strategy to maximise vaccine immunogenicity. Here, we conducted a phase I, randomised control trial to observe the safety and immunogenicity after an intradermal boost, using a fractional dosage (1:5) of BNT162b2 mRNA vaccine in healthy participants in Songkhla, Thailand. In total, 91 volunteers who had been administered with two doses of inactivated SARS-CoV-2 (CoronaVac) were recruited into the study, and then randomised (1:1:1) to received different regimens of the third dose. An intramuscular booster with a full dose of BNT162b2 was included as a conventional control, and a half dose group was included as reciprocal comparator. Both, immediate and delayed adverse events following immunisation (AEFI) were monitored. Humoral and cellular immune responses were examined to observe the booster effects. The intradermal booster provided significantly fewer systemic side effects, from 70% down to 19.4% (p < 0.001); however, they were comparable to local reactions with the conventional intramuscular booster. In the intradermal group after receiving only one fifth of the conventional dosage, serum Anti-RBD IgG was halved compared to the full dose of an intramuscular injection. However, the neutralising function against the Delta strain remained intact. T cell responses were also less effective in the intradermal group compared to the intramuscular booster. Together, the intradermal booster, using a fractional dose of BNT162b2, can reduce systemic reactions and provides a good level and function of antibody responses compared to the conventional booster. This favourable intradermal boosting strategy provides a suitable alternative for vaccines and effective vaccine management to increase the coverage during the vaccine shortage.
Keywords: COVID-19; immunogenicity; inactivated SARS-CoV-2; intradermal; mRNA vaccine.
Conflict of interest statement
The authors declare no Competing Financial or Non-Financial Interests.
Figures
References
-
- European Centre for Disease Prevention and Control . Threat Assessment Brief: Emergence of SARS CoV 2 B. 1.617 Variants in India and Situation in the EU/EEA. European Centre for Disease Prevention and Control; Stockholm, Sweden: 2021.
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

