PICK-ing Malaysia's Epidemic Apart: Effectiveness of a Diverse COVID-19 Vaccine Portfolio
- PMID: 34960126
- PMCID: PMC8706086
- DOI: 10.3390/vaccines9121381
PICK-ing Malaysia's Epidemic Apart: Effectiveness of a Diverse COVID-19 Vaccine Portfolio
Abstract
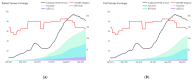
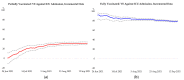
Malaysia rolled out a diverse portfolio of predominantly three COVID-19 vaccines (AZD1222, BNT162b2, and CoronaVac) beginning 24 February 2021. We evaluated vaccine effectiveness with two methods, covering 1 April to 15 September 2021: (1) the screening method for COVID-19 (SARS-CoV-2) infection and symptomatic COVID-19; and (2) a retrospective cohort of confirmed COVID-19 cases for COVID-19 related ICU admission and death using logistic regression. The screening method estimated partial vaccination to be 48.8% effective (95% CI: 46.8, 50.7) against COVID-19 infection and 33.5% effective (95% CI: 31.6, 35.5) against symptomatic COVID-19. Full vaccination is estimated at 87.8% effective (95% CI: 85.8, 89.7) against COVID-19 infection and 85.4% effective (95% CI: 83.4, 87.3) against symptomatic COVID-19. Among the cohort of confirmed COVID-19 cases, partial vaccination with any of the three vaccines is estimated at 31.3% effective (95% CI: 28.5, 34.1) in preventing ICU admission, and 45.1% effective (95% CI: 42.6, 47.5) in preventing death. Full vaccination with any of the three vaccines is estimated at 79.1% effective (95% CI: 77.7, 80.4) in preventing ICU admission and 86.7% effective (95% CI: 85.7, 87.6) in preventing deaths. Our findings suggest that full vaccination with any of the three predominant vaccines (AZD1222, BNT162b2, and CoronaVac) in Malaysia has been highly effective in preventing COVID-19 infection, symptomatic COVID-19, COVID-19-related ICU admission, and death.
Keywords: COVID-19; COVID-19 vaccines; Malaysia; SARS-CoV-2; cohort study; vaccine effectiveness.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures



References
-
- Cascella M., Rajnik M., Aleem A., Dulebohn S.C., Di Napoli R. StatPearls [Internet] StatPearls Publishing; Treasure Island, FL, USA: 2021. Features, Evaluation, and Treatment of Coronavirus (COVID-19) - PubMed
-
- World Health Organization COVID-19 Weekly Epidemiological Update–Edition 64. [(accessed on 2 November 2021)]. Available online: https://www.who.int/publications/m/item/weekly-epidemiological-update-on....
-
- World Health Organization COVID-19 Vaccine Tracker and Landscape. [(accessed on 26 September 2021)]. Available online: https://www.who.int/publications/m/item/draft-landscape-of-covid-19-cand....
LinkOut - more resources
Full Text Sources
Miscellaneous

