Directing T-Cell Immune Responses for Cancer Vaccination and Immunotherapy
- PMID: 34960140
- PMCID: PMC8708201
- DOI: 10.3390/vaccines9121392
Directing T-Cell Immune Responses for Cancer Vaccination and Immunotherapy
Abstract
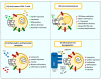
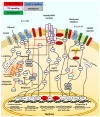
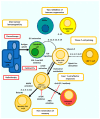
Cancer vaccination and immunotherapy revolutionised the treatment of cancer, a result of decades of research into the immune system in health and disease. However, despite recent breakthroughs in treating otherwise terminal cancer, only a minority of patients respond to cancer immunotherapy and some cancers are largely refractive to immunotherapy treatment. This is due to numerous issues intrinsic to the tumour, its microenvironment, or the immune system. CD4+ and CD8+ αβ T-cells emerged as the primary effector cells of the anti-tumour immune response but their function in cancer patients is often compromised. This review details the mechanisms by which T-cell responses are hindered in the setting of cancer and refractive to immunotherapy, and details many of the approaches under investigation to direct T-cell function and improve the efficacy of cancer vaccination and immunotherapy.
Keywords: T-cell; cancer; checkpoint inhibition; immunotherapy; metabolism; microbiome; vaccine.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Revisiting the role of CD4+ T cells in cancer immunotherapy-new insights into old paradigms.Cancer Gene Ther. 2021 Feb;28(1-2):5-17. doi: 10.1038/s41417-020-0183-x. Epub 2020 May 27. Cancer Gene Ther. 2021. PMID: 32457487 Free PMC article. Review.
-
CD4+ T-cell epitope-based heterologous prime-boost vaccination potentiates anti-tumor immunity and PD-1/PD-L1 immunotherapy.J Immunother Cancer. 2022 May;10(5):e004022. doi: 10.1136/jitc-2021-004022. J Immunother Cancer. 2022. PMID: 35580929 Free PMC article.
-
Directing the Future Breakthroughs in Immunotherapy: The Importance of a Holistic Approach to the Tumour Microenvironment.Cancers (Basel). 2021 Nov 24;13(23):5911. doi: 10.3390/cancers13235911. Cancers (Basel). 2021. PMID: 34885021 Free PMC article. Review.
-
Therapeutic vaccination immunomodulation: forming the basis of all cancer immunotherapy.Ther Adv Vaccines Immunother. 2019 Aug 1;7:2515135519862234. doi: 10.1177/2515135519862234. eCollection 2019. Ther Adv Vaccines Immunother. 2019. PMID: 31414074 Free PMC article. Review.
-
The implication of anti-PD-1 therapy in cancer patients for the vaccination against viral and other infectious diseases.Pharmacol Ther. 2023 May;245:108399. doi: 10.1016/j.pharmthera.2023.108399. Epub 2023 Mar 30. Pharmacol Ther. 2023. PMID: 37001736 Review.
Cited by
-
Cancer theragnostics: closing the loop for advanced personalized cancer treatment through the platform integration of therapeutics and diagnostics.Front Bioeng Biotechnol. 2025 Jan 17;12:1499474. doi: 10.3389/fbioe.2024.1499474. eCollection 2024. Front Bioeng Biotechnol. 2025. PMID: 39898278 Free PMC article. Review.
-
Enhancement of subunit vaccine delivery with zinc-carnosine coordination polymer through the addition of mannan.Int J Pharm. 2024 May 10;656:124076. doi: 10.1016/j.ijpharm.2024.124076. Epub 2024 Apr 1. Int J Pharm. 2024. PMID: 38569976 Free PMC article.
-
Current Immunotherapy Treatments of Primary Breast Cancer Subtypes.Biomedicines. 2024 Apr 18;12(4):895. doi: 10.3390/biomedicines12040895. Biomedicines. 2024. PMID: 38672249 Free PMC article. Review.
-
Advances in the Immunomodulatory Properties of Glycoantigens in Cancer.Cancers (Basel). 2022 Apr 7;14(8):1854. doi: 10.3390/cancers14081854. Cancers (Basel). 2022. PMID: 35454762 Free PMC article. Review.
-
Immunotherapy in breast cancer: current landscape and emerging trends.Exp Hematol Oncol. 2025 May 22;14(1):77. doi: 10.1186/s40164-025-00667-y. Exp Hematol Oncol. 2025. PMID: 40405250 Free PMC article. Review.
References
-
- Kusnadi A., Ramírez-Suástegui C., Fajardo V., Chee S.J., Meckiff B.J., Simon H., Pelosi E., Seumois G., Ay F., Vijayanand P., et al. Severely ill COVID-19 patients display impaired exhaustion features in SARS-CoV-2-reactive CD8+ T cells. Sci. Immunol. 2021;6:eabe4782. doi: 10.1126/sciimmunol.abe4782. - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials

