Longitudinal Characterization of the Mumps-Specific HLA-A2 Restricted T-Cell Response after Mumps Virus Infection
- PMID: 34960178
- PMCID: PMC8707000
- DOI: 10.3390/vaccines9121431
Longitudinal Characterization of the Mumps-Specific HLA-A2 Restricted T-Cell Response after Mumps Virus Infection
Abstract
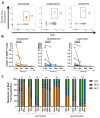
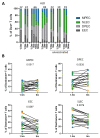
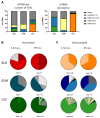
Waning of the mumps virus (MuV)-specific humoral response after vaccination has been suggested as a cause for recent mumps outbreaks in vaccinated young adults, although it cannot explain all cases. Moreover, CD8+ T cells may play an important role in the response against MuV; however, little is known about the characteristics and dynamics of the MuV-specific CD8+ T-cell response after MuV infection. Here, we had the opportunity to follow the CD8+ T-cell response to three recently identified HLA-A2*02:01-restricted MuV-specific epitopes from 1.5 to 36 months post-MuV infection in five previously vaccinated and three unvaccinated individuals. The infection-induced CD8+ T-cell response was dominated by T cells specific for the ALDQTDIRV and LLDSSTTRV epitopes, while the response to the GLMEGQIVSV epitope was subdominant. MuV-specific CD8+ T-cell frequencies in the blood declined between 1.5 and 9 months after infection. This decline was not explained by changes in the expression of inhibitory receptors or homing markers. Despite the ongoing changes in the frequencies and phenotype of MuV-specific CD8+ T cells, TCRβ analyses revealed a stable MuV-specific T-cell repertoire over time. These insights in the maintenance of the cellular response against mumps may provide hallmarks for optimizing vaccination strategies towards a long-term cellular memory response.
Keywords: MMR vaccination; T-cell immunity; mumps infection.
Conflict of interest statement
The authors declare no competing interests.
Figures

References
-
- MacDonald N., Hatchette T., Elkout L., Sarwal S. Mumps is Back: Why is Mumps Eradication Not Working? Adv. Exp. Med. Biol. 2011;7:197–220. - PubMed
-
- Plotkin S.A., Orenstein W.A., Offit P.A. Vaccines. Elsevier; Philadelphia, PA, USA: 2018.
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

