Vaccine Procurement: A Conceptual Framework Based on Literature Review
- PMID: 34960180
- PMCID: PMC8707219
- DOI: 10.3390/vaccines9121434
Vaccine Procurement: A Conceptual Framework Based on Literature Review
Abstract
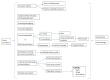
Ensuring timely access to affordable vaccines has been acknowledged as a global public health priority, as also recently testified by the debate sparked during the COVID-19 pandemic. Effective vaccine procurement strategies are essential to reach this goal. Nevertheless, this is still a neglected research topic. A narrative literature review on vaccine procurement was conducted, by retrieving articles from four academic databases (PubMed/MEDLINE, Scopus, Embase, WebOfScience), 'grey' literature reports, and institutional websites. The aim was to clarify key concepts and definitions relating to vaccine procurement, describe main vaccine procurement methods, and identify knowledge gaps and future perspectives. A theoretical conceptual framework was developed of the key factors involved in vaccine procurement, which include quality and safety of the product, forecasting and budgeting, procurement legislation, financial sustainability, and plurality of manufacture, contracting, investment in training, storage and service delivery, monitoring and evaluation. This information can be useful to support policymakers during planning, implementation, and evaluation of regional and national vaccine procurement strategies and policies.
Keywords: Europe; immunization; review; vaccine; vaccine procurement.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Robson M., Andrus J.K., Toscano C.M., Lewis M., Oliveira L., Ropero A.M., Davila M., Fitzsimmons J.W. A model for enhancing evidence-based capacity to make informed policy decisions on the introduction of new vaccines in the Americas: PAHO’s ProVac initiative. Public Health Rep. 2007;122:811–816. doi: 10.1177/003335490712200613. - DOI - PMC - PubMed
-
- Pubmed/National Library of Medicine’s Publication Characteristics (Publication Types) with Scope Notes. [(accessed on 3 March 2020)];2020 Available online: https://www.nlm.nih.gov/mesh/pubtypes.html.