Nasal and Salivary Mucosal Humoral Immune Response Elicited by mRNA BNT162b2 COVID-19 Vaccine Compared to SARS-CoV-2 Natural Infection
- PMID: 34960244
- PMCID: PMC8708818
- DOI: 10.3390/vaccines9121499
Nasal and Salivary Mucosal Humoral Immune Response Elicited by mRNA BNT162b2 COVID-19 Vaccine Compared to SARS-CoV-2 Natural Infection
Erratum in
-
Correction: Guerrieri et al. Nasal and Salivary Mucosal Humoral Immune Response Elicited by mRNA BNT162b2 COVID-19 Vaccine Compared to SARS-CoV-2 Natural Infection. Vaccines 2021, 9, 1499.Vaccines (Basel). 2023 Jan 13;11(1):172. doi: 10.3390/vaccines11010172. Vaccines (Basel). 2023. PMID: 36680041 Free PMC article.
Abstract
SARS-CoV-2 antibody assays are crucial in managing the COVID-19 pandemic. Approved mRNA COVID-19 vaccines are well known to induce a serum antibody responses against the spike protein and its RBD. Mucosal immunity plays a major role in the fight against COVID-19 directly at the site of virus entry; however, vaccine abilities to elicit mucosal immune responses have not been reported. We detected anti-SARS-CoV-2 IgA-S1 and IgG-RBD in three study populations (healthy controls, vaccinated subjects, and subjects recovered from COVID-19 infection) on serum, saliva, and nasal secretions using two commercial immunoassays (ELISA for IgA-S1 and chemiluminescent assay for IgG-RBD). Our results show that the mRNA BNT162b2 vaccine Comirnaty (Pfizer/BioNTech, New York, NY, USA) determines the production of nasal and salivary IgA-S1 and IgG-RBD against SARS-CoV-2. This mucosal humoral immune response is stronger after the injection of the second vaccine dose compared to subjects recovered from COVID-19. Since there is a lack of validated assays on saliva and nasal secretions, this study shows that our pre-analytical and analytical procedures are consistent with the data. Our findings indicate that the mRNA COVID-19 vaccine elicits antigen-specific nasal and salivary immune responses, and that mucosal antibody assays could be used as candidates for non-invasive monitoring of vaccine-induced protection against viral infection.
Keywords: BNT162b2; COVID-19; IgA; IgG-RBD; SARS-CoV-2; immunity; mucosal; nasal; salivary; vaccine.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

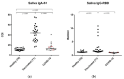

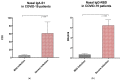
References
-
- Cervia C., Nilsson J., Zurbuchen Y., Valaperti A., Schreiner J., Wolfensberger A., Raeber M.E., Adamo S., Weigang S., Emmenegger M., et al. Systemic and mucosal antibody responses specific to SARS-CoV-2 during mild versus severe COVID-19. J. Allergy Clin. Immunol. 2021;147:545–557.e9. doi: 10.1016/j.jaci.2020.10.040. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

