Chitosan Nanoparticles Inactivate Alfalfa Mosaic Virus Replication and Boost Innate Immunity in Nicotiana glutinosa Plants
- PMID: 34961172
- PMCID: PMC8703458
- DOI: 10.3390/plants10122701
Chitosan Nanoparticles Inactivate Alfalfa Mosaic Virus Replication and Boost Innate Immunity in Nicotiana glutinosa Plants
Abstract
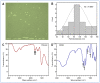
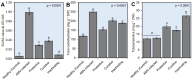
Plant viral infection is one of the most severe issues in food security globally, resulting in considerable crop production losses. Chitosan is a well-known biocontrol agent against a variety of plant infections. However, research on combatting viral infections is still in its early stages. The current study investigated the antiviral activities (protective, curative, and inactivation) of the prepared chitosan/dextran nanoparticles (CDNPs, 100 µg mL-1) on Nicotiana glutinosa plants. Scanning electron microscope (SEM) and dynamic light scattering analysis revealed that the synthesized CDNPs had a uniform, regular sphere shapes ranging from 20 to 160 nm in diameter, with an average diameter of 91.68 nm. The inactivation treatment was the most effective treatment, which resulted in a 100% reduction in the alfalfa mosaic virus (AMV, Acc# OK413670) accumulation level. On the other hand, the foliar application of CDNPs decreased disease severity and significantly reduced viral accumulation levels by 70.43% and 61.65% in protective and curative treatments, respectively, under greenhouse conditions. Additionally, the induction of systemic acquired resistance, increasing total carbohydrates and total phenolic contents, as well as triggering the transcriptional levels of peroxidase, pathogen-related protein-1, and phenylalanine ammonia-lyase were observed. In light of the results, we propose that the potential application of CDNPs could be an eco-friendly approach to enhance yield and a more effective therapeutic elicitor for disease management in plants upon induction of defense systems.
Keywords: alfalfa mosaic virus; antiviral activity; chitosan nanoparticles; gene expression.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






References
-
- Abdelkhalek A., Hafez E. Cottage Industry of Biocontrol Agents and Their Applications. Springer; Berlin/Heidelberg, Germany: 2020. Plant Viral Diseases in Egypt and Their Control; pp. 403–421.
-
- El-Helaly H.S., Ahmed A.A., Awad M.A., Soliman A.M. Biological and molecular characterization of potato infecting alfalfa mosaic virus in Egypt. Int. J. Virol. 2012;8:106–113. doi: 10.3923/ijv.2012.106.113. - DOI
-
- Mangeli F., Massumi H., Alipour F., Maddahian M., Heydarnejad J., Hosseinipour A., Amid-Motlagh M.H., Azizizadeh M., Varsani A. Molecular and partial biological characterization of the coat protein sequences of Iranian alfalfa mosaic virus isolates. J. Plant Pathol. 2019;101:735–742. doi: 10.1007/s42161-019-00275-w. - DOI
-
- Trucco V., Castellanos Collazo O., Vaghi Medina C.G., Cabrera Mederos D., Lenardon S., Giolitti F. Alfalfa mosaic virus (AMV): Genetic diversity and a new natural host. J. Plant Pathol. 2021:1–8. doi: 10.1007/s42161-021-00961-8. - DOI
LinkOut - more resources
Full Text Sources
Research Materials

