New benzoxazole derivatives as potential VEGFR-2 inhibitors and apoptosis inducers: design, synthesis, anti-proliferative evaluation, flowcytometric analysis, and in silico studies
- PMID: 34961427
- PMCID: PMC8725875
- DOI: 10.1080/14756366.2021.2015343
New benzoxazole derivatives as potential VEGFR-2 inhibitors and apoptosis inducers: design, synthesis, anti-proliferative evaluation, flowcytometric analysis, and in silico studies
Erratum in
-
Correction.J Enzyme Inhib Med Chem. 2022 Dec;37(1):514. doi: 10.1080/14756366.2022.2024999. J Enzyme Inhib Med Chem. 2022. PMID: 34986713 Free PMC article. No abstract available.
Abstract
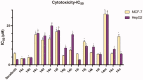
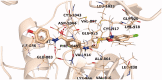
A new series of benzoxazole derivatives were designed and synthesised to have the main essential pharmacophoric features of VEGFR-2 inhibitors. Cytotoxic activities were evaluated for all derivatives against two human cancer cell lines, MCF-7 and HepG2. Also, the effect of the most cytotoxic derivatives on VEGFR-2 protein concentration was assessed by ELISA. Compounds 14o, 14l, and 14b showed the highest activities with VEGFR-2 protein concentrations of 586.3, 636.2, and 705.7 pg/ml, respectively. Additionally, the anti-angiogenic property of compound 14b against human umbilical vascular endothelial cell (HUVEC) was performed using a wound healing migration assay. Compound 14b reduced proliferation and migratory potential of HUVEC cells. Furthermore, compound 14b was subjected to further biological investigations including cell cycle and apoptosis analyses. Compound 14b arrested the HepG2 cell growth at the Pre-G1 phase and induced apoptosis by 16.52%, compared to 0.67% in the control (HepG2) cells. The effect of apoptosis was buttressed by a 4.8-fold increase in caspase-3 level compared to the control cells. Besides, different in silico docking studies were also performed to get better insights into the possible binding mode of the target compounds with VEGFR-2 active sites.
Keywords: Anti-proliferative; VEGFR-2 inhibitors; apoptosis; benzoxazole.
Conflict of interest statement
No potential conflict of interest was reported by the authors.
Figures














References
-
- Folkman J. Angiogenesis in cancer, vascular, rheumatoid and other disease. Nat Med 1995;1:27–30. - PubMed
-
- Kerbel RS. Tumor angiogenesis: past, present and the near future. Carcinogenesis 2000;21:505–15. - PubMed
-
- Behdani M, Zeinali S, Khanahmad H, et al. . Generation and characterization of a functional nanobody against the vascular endothelial growth factor receptor-2; angiogenesis cell receptor. Mol Immunol 2012;50:35–41. - PubMed
-
- Lee YT, Tan YJ, Oon CE.. Molecular targeted therapy: treating cancer with specificity. Eur J Pharmacol 2018;834:188–96. - PubMed
-
- Chen H, Kovar J, Sissons S, et al. . A cell-based immunocytochemical assay for monitoring kinase signaling pathways and drug efficacy. Anal Biochem 2005;338:136–42. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
