Methodological approaches for conducting follow-up research with clinical trial participants: a scoping review and expert interviews
- PMID: 34961543
- PMCID: PMC8711196
- DOI: 10.1186/s13063-021-05866-6
Methodological approaches for conducting follow-up research with clinical trial participants: a scoping review and expert interviews
Abstract
Background: Evidence-based establishment and implementation of best principles, laws and ordinances that regulate clinical research depend on the consultation and involvement of trial participants. Yet, guidance on methodological approaches to obtain trial participants' perspectives is currently missing. This scoping review therefore aimed at identifying, describing and evaluating research approaches to obtain trial participants' feedback on their views and experiences.
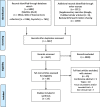
Methods: We searched the electronic databases Medline and PsycInfo via Ovid and the Web of Science Core Collection. Clinical trials were included that involved adult participants that were conducted in selected high-income countries and that were published in peer-reviewed journals between 1985 and 2018. In addition, 29 expert interviews were conducted between March and May 2019.
Results: Out of 5994 identified records, 23 articles were included in this review. Twelve studies used a qualitative approach, 10 were quantitative and one study used a mixed-method design. More than 75% of all work was conducted in the USA and the UK. The scoping review and the expert interviews highlighted that recruitment of participants was generally done through direct contact by principal investigators and/or study nurses or through searches in de-identified patient databases. Authors used surveys, interviews or focus group discussions. The tools used were either based on existing validated ones or developed and verified de novo with the support of experts and/or patient representatives.
Conclusions: To our knowledge, this is the first methodological literature review of approaches to researching experiences of clinical trial participants where findings were triangulated with expert interviews. Covering a range of indications, trial phases and study settings, it demonstrates that clinical trial participant perspectives and experience is heavily under-researched. This casts doubt on the overall robustness of available insight into trial participants' views and experiences. Our results demonstrate that the methodology for studying participant opinion, perception and experience should be adapted to the measure of interest and conform to the study population. Using valid patient experience data is the basis to evaluate existing legal and regulatory human subject research frameworks for their appropriateness from a patient perspective. Such an evaluation will be critical to empower research participants.
Keywords: Methodological approach; Patient experience; Recruitment; Trials.
© 2021. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
References
-
- Joffe S, Cook EF, Cleary PD, Clark JW, Weeks JC. Quality of informed consent in cancer clinical trials: a cross-sectional survey. Lancet. 2001;358(9295):1772–1777. - PubMed
-
- Burks AC, Keim-Malpass J. Health literacy and informed consent for clinical trials: a systematic review and implications for nurses. Nursing-Res Rev. 2019;9:31–40.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources