Mono-ADP-ribosylation sites of human CD73 inhibit its adenosine-generating enzymatic activity
- PMID: 34961895
- PMCID: PMC8850506
- DOI: 10.1007/s11302-021-09832-4
Mono-ADP-ribosylation sites of human CD73 inhibit its adenosine-generating enzymatic activity
Abstract
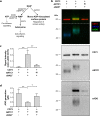
CD73-derived adenosine plays a major role in damage-induced tissue responses by inhibiting inflammation. Damage-associated stimuli, such as hypoxia and mechanical stress, induce the cellular release of ATP and NAD+ and upregulate the expression of the nucleotide-degrading purinergic ectoenzyme cascade, including adenosine-generating CD73. Extracellular NAD+ also serves as substrate for mono-ADP-ribosylation of cell surface proteins, which in human cells is mediated by ecto-ADP-ribosyltransferase 1 (ARTC1). Here we explored, whether human CD73 enzymatic activity is regulated by mono-ADP-ribosylation, using recombinant human CD73 in the presence of ARTC1 with etheno-labelled NAD+ as substrate. Multi-colour immunoblotting with an anti-etheno-adenosine antibody showed ARTC1-mediated transfer of ADP-ribose together with the etheno label to CD73. HPLC analysis of the enzymatic activity of in vitro-ribosylated CD73 revealed strong inhibition of adenosine generation in comparison to non-ribosylated CD73. Mass spectrometry of in vitro-ribosylated CD73 identified six ribosylation sites. 3D model analysis indicated that three of them (R328, R354, R545) can interfere with CD73 enzymatic activity. Our study identifies human CD73 as target for ARTC1-mediated mono-ADP-ribosylation, which can profoundly modulate its adenosine-generating activity. Thus, in settings with enhanced release of NAD+ as substrate for ARTC1, assessment of CD73 protein expression in human tissues may not be predictive of adenosine formation resulting in anti-inflammatory activity.
Keywords: Adenosine; CD296; Mono-ADP-ribosylation; NT5e; Post-translational modification.
© 2021. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

