Brand-Specific Enhanced Safety Surveillance Study of GSK's Quadrivalent Seasonal Influenza Vaccine, Conducted During the COVID-19 Pandemic, in Belgium, Germany and Spain, for the 2020/21 Season
- PMID: 34961900
- PMCID: PMC8711683
- DOI: 10.1007/s40121-021-00571-y
Brand-Specific Enhanced Safety Surveillance Study of GSK's Quadrivalent Seasonal Influenza Vaccine, Conducted During the COVID-19 Pandemic, in Belgium, Germany and Spain, for the 2020/21 Season
Abstract
Introduction: Seasonal influenza poses a major public health burden worldwide. Influenza vaccines, updated yearly to match circulating strains based on World Health Organization (WHO) recommendations, are the cornerstone of prevention and require regular monitoring. The COVID-19 pandemic is expected to cause logistical, site access and medical staff constraints and could affect the safety profile of influenza vaccines.
Methods: Following European Medicines Agency guidance, an enhanced safety surveillance (ESS) study assessed the frequency and severity of predefined and other adverse events (AEs) occurring within 7 days of receiving GSK's inactivated quadrivalent seasonal influenza vaccine (IIV4), in Belgium, Germany and Spain in 2020/21, using adverse drug reaction (ADR) cards.
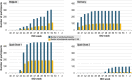
Results: During the 2020/21 influenza season, 1054 participants vaccinated with GSK's IIV4 were enrolled (all adults in Belgium and Germany, 30% adults/70% children in Spain); 96 eligible children received a second dose. Overall, 1042 participants completed the study. After doses 1 and 2, 98.9% and 100% of participants, respectively, returned their completed ADR card. After doses 1 and 2, 37.8% (398/1054) and 13.5% (13/96) of participants, respectively, reported at least one AE. The most frequently reported categories of AEs were "general disorders and administration site conditions" (e.g. injection site pain) and "nervous system disorders" (e.g. headache). There were no deaths or serious AEs deemed related to GSK's IIV4.
Conclusion: This ESS study assessed AEs in near real time. The COVID-19 pandemic did not alter the safety profile of GSK's IIV4. No safety signals were detected during the study, which confirms the excellent safety profile of GSK's IIV4.
Keywords: AlphaRix Tetra; Fluarix Tetra; Infectious disease; Influenza; Influsplit Tetra; Post-marketing surveillance; Vaccination; Vaccine Safety; Vaccine monitoring.
© 2021. The GSK group of companies.
Figures

References
-
- European Centre for Disease Prevention and Control (ECDC). Factsheet about seasonal influenza. https://ecdc.europa.eu/en/seasonal-influenza/facts/factsheet. Accessed 1 Jul 2021.
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

