Novel cannula design improves large volume auto-injection rates for high viscosity solutions
- PMID: 34962225
- PMCID: PMC8725910
- DOI: 10.1080/10717544.2021.2018069
Novel cannula design improves large volume auto-injection rates for high viscosity solutions
Abstract
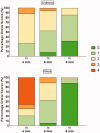
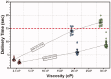
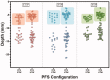
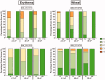
A prototype reusable large-volume (2 mL) autoinjector (LVAI) was designed to compare injection performance of a novel 27 gauge ultra-thin wall (UTW) pre-filled syringe (PFS) cannula (8 mm external cannula length, 14.4 mm total needle length) against an existing 27 gauge special thin wall (STW) PFS cannula (12.7 mm external cannula length, 19 mm total needle length) across a range of injectate viscosities (2.3-30 cP) in a series of in vivo feasibility studies in swine. The UTW cannula had an approximately 30% greater cross-sectional lumen area than the STW cannula. The target exposed needle length was adjusted to ensure appropriate needle penetration depth and achieve injectate deposition in the subcutaneous (SC) tissue. Delivery time and volume, injection site leakage, injectate depot location, and local tissue effects were examined. The STW and UTW cannulae both provided effective SC delivery of contrast placebo solutions, and were able to accommodate injectate viscosity up to 30 cP without quantifiable leakage from the tissue and with minor tissue effects which resolved within 1-2 hours. Delivery times at each viscosity were significantly different between PFS types with the UTW PFS producing faster delivery times. In a histological substudy of the UTW cannula using injectate viscosities up to 50 cP, injection site reactions were rare and, when present, were of minimal severity. This series of studies demonstrates the feasibility of LVAI SC injection and informs autoinjector and PFS design considerations. Use of a UTW cannula may enable more rapid LVAI injections with minimal tissue effects, especially for higher viscosity formulations.
Keywords: Large volume subcutaneous injection; autoinjector; high viscosity; injection depth; in vivo; pre-filled syringe; tissue response; ultra-thin wall cannula.
Conflict of interest statement
Bruce Roberts, Christopher Rini, Rick Klug, Douglas B. Sherman, Didier Morel, and Ronald J. Pettis are employees and potential stockholders of BD, which sponsored this work.
Figures




References
-
- Abolhassani H, Sadaghiani MS, Aghamohammadi A, et al. (2012). Home-based subcutaneous immunoglobulin versus hospital-based intravenous immunoglobulin in treatment of primary antibody deficiencies: systematic review and meta analysis. J Clin Immunol 32:1180–92. - PubMed
-
- Allmendinger A, Fischer S, Huwyler J, et al. (2014). Rheological characterization and injection forces of concentrated protein formulations: an alternative predictive model for non-Newtonian solutions. Eur J Pharm Biopharm 87:318–28. - PubMed
-
- Allmendinger A, Mueller R, Schwarb E, et al. (2015). Measuring tissue back-pressure—in vivo injection forces during subcutaneous injection. Pharm Res 32:2229–40. - PubMed
-
- Bernstein D, Pavord ID, Chapman KR, et al. (2020). Usability of mepolizumab single-use prefilled autoinjector for patient self-administration. J Asthma 57:987–98. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
