Association Between Immune Dysfunction and COVID-19 Breakthrough Infection After SARS-CoV-2 Vaccination in the US
- PMID: 34962505
- PMCID: PMC8715386
- DOI: 10.1001/jamainternmed.2021.7024
Association Between Immune Dysfunction and COVID-19 Breakthrough Infection After SARS-CoV-2 Vaccination in the US
Abstract
Importance: Persons with immune dysfunction have a higher risk for severe COVID-19 outcomes. However, these patients were largely excluded from SARS-CoV-2 vaccine clinical trials, creating a large evidence gap.
Objective: To identify the incidence rate and incidence rate ratio (IRR) for COVID-19 breakthrough infection after SARS-CoV-2 vaccination among persons with or without immune dysfunction.
Design, setting, and participants: This retrospective cohort study analyzed data from the National COVID Cohort Collaborative (N3C), a partnership that developed a secure, centralized electronic medical record-based repository of COVID-19 clinical data from academic medical centers across the US. Persons who received at least 1 dose of a SARS-CoV-2 vaccine between December 10, 2020, and September 16, 2021, were included in the sample.
Main outcomes and measures: Vaccination, COVID-19 diagnosis, immune dysfunction diagnoses (ie, HIV infection, multiple sclerosis, rheumatoid arthritis, solid organ transplant, and bone marrow transplantation), other comorbid conditions, and demographic data were accessed through the N3C Data Enclave. Breakthrough infection was defined as a COVID-19 infection that was contracted on or after the 14th day of vaccination, and the risk after full or partial vaccination was assessed for patients with or without immune dysfunction using Poisson regression with robust SEs. Poisson regression models were controlled for a study period (before or after [pre- or post-Delta variant] June 20, 2021), full vaccination status, COVID-19 infection before vaccination, demographic characteristics, geographic location, and comorbidity burden.
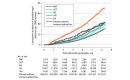
Results: A total of 664 722 patients in the N3C sample were included. These patients had a median (IQR) age of 51 (34-66) years and were predominantly women (n = 378 307 [56.9%]). Overall, the incidence rate for COVID-19 breakthrough infection was 5.0 per 1000 person-months among fully vaccinated persons but was higher after the Delta variant became the dominant SARS-CoV-2 strain (incidence rate before vs after June 20, 2021, 2.2 [95% CI, 2.2-2.2] vs 7.3 [95% CI, 7.3-7.4] per 1000 person-months). Compared with partial vaccination, full vaccination was associated with a 28% reduced risk for breakthrough infection (adjusted IRR [AIRR], 0.72; 95% CI, 0.68-0.76). People with a breakthrough infection after full vaccination were more likely to be older and women. People with HIV infection (AIRR, 1.33; 95% CI, 1.18-1.49), rheumatoid arthritis (AIRR, 1.20; 95% CI, 1.09-1.32), and solid organ transplant (AIRR, 2.16; 95% CI, 1.96-2.38) had a higher rate of breakthrough infection.
Conclusions and relevance: This cohort study found that full vaccination was associated with reduced risk of COVID-19 breakthrough infection, regardless of the immune status of patients. Despite full vaccination, persons with immune dysfunction had substantially higher risk for COVID-19 breakthrough infection than those without such a condition. For persons with immune dysfunction, continued use of nonpharmaceutical interventions (eg, mask wearing) and alternative vaccine strategies (eg, additional doses or immunogenicity testing) are recommended even after full vaccination.
Conflict of interest statement
Figures

Comment in
-
COVID-19 Breakthrough Infection Among Immunocompromised Persons.JAMA Intern Med. 2022 Feb 1;182(2):163-164. doi: 10.1001/jamainternmed.2021.7033. JAMA Intern Med. 2022. PMID: 34962552 No abstract available.
References
-
- Abu Jabal K, Ben-Amram H, Beiruti K, et al. Impact of age, ethnicity, sex and prior infection status on immunogenicity following a single dose of the BNT162b2 mRNA COVID-19 vaccine: real-world evidence from healthcare workers, Israel, December 2020 to January 2021. Euro Surveill. 2021;26(6):2100096. doi: 10.2807/1560-7917.ES.2021.26.6.2100096 - DOI - PMC - PubMed
-
- Haas EJ, Angulo FJ, McLaughlin JM, et al. Impact and effectiveness of mRNA BNT162b2 vaccine against SARS-CoV-2 infections and COVID-19 cases, hospitalisations, and deaths following a nationwide vaccination campaign in Israel: an observational study using national surveillance data. Lancet. 2021;397(10287):1819-1829. doi: 10.1016/S0140-6736(21)00947-8 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

