Developing a reproducible protocol for culturing functional confluent monolayers of differentiated equine oviduct epithelial cells†
- PMID: 34962550
- PMCID: PMC9040661
- DOI: 10.1093/biolre/ioab243
Developing a reproducible protocol for culturing functional confluent monolayers of differentiated equine oviduct epithelial cells†
Abstract
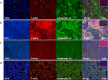
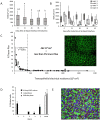
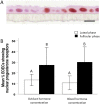
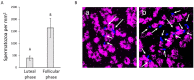
We describe the development of two methods for obtaining confluent monolayers of polarized, differentiated equine oviduct epithelial cells (EOEC) in Transwell inserts and microfluidic chips. EOECs from the ampulla were isolated post-mortem and seeded either (1) directly onto a microporous membrane as differentiated EOECs (direct seeding protocol) or (2) first cultured to a confluent de-differentiated monolayer in conventional wells, then trypsinized and seeded onto a microporous membrane (re-differentiation protocol). Maintenance or induction of EOEC differentiation in these systems was achieved by air-liquid interface introduction. Monolayers cultured via both protocols were characterized by columnar, cytokeratin 19-positive EOECs in Transwell inserts. However, only the re-differentiation protocol could be transferred successfully to the microfluidic chips. Integrity of the monolayers was confirmed by transepithelial resistance measurements, tracer flux, and the demonstration of an intimate network of tight junctions. Using the direct protocol, 28% of EOECs showed secondary cilia at the apical surface in a diffuse pattern. In contrast, re-differentiated polarized EOECs rarely showed secondary cilia in either culture system (>90% of the monolayers showed <1% ciliated EOECs). Occasionally (5-10%), re-differentiated monolayers with 11-27% EOECs with secondary cilia in a diffuse pattern were obtained. Additionally, nuclear progesterone receptor expression was found to be inhibited by simulated luteal phase hormone concentrations, and sperm binding to cilia was higher for re-differentiated EOEC monolayers exposed to estrogen-progesterone concentrations mimicking the follicular rather than luteal phase. Overall, a functional equine oviduct model was established with close morphological resemblance to in vivo oviduct epithelium.
Keywords: Transwell culture; cilia; equine; microfluidic chip; oviduct; primary cell culture.
© The Author(s) 2021. Published by Oxford University Press on behalf of Society for the Study of Reproduction. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Figures





Similar articles
-
Induction of in vivo-like ciliation in confluent monolayers of re-differentiated equine oviduct epithelial cells†.Biol Reprod. 2024 Sep 14;111(3):580-599. doi: 10.1093/biolre/ioae090. Biol Reprod. 2024. PMID: 38847468 Free PMC article.
-
Culture of bovine oviduct epithelial cells (BOEC).Anat Rec. 1995 Nov;243(3):347-56. doi: 10.1002/ar.1092430309. Anat Rec. 1995. PMID: 8579254
-
S-100 protein subunits in bovine oviduct epithelium: in situ distribution and changes during primary cell culture.Histochem J. 1996 Oct;28(10):671-80. doi: 10.1007/BF02409004. Histochem J. 1996. PMID: 8950596
-
Physiological Action of Progesterone in the Nonhuman Primate Oviduct.Cells. 2022 May 3;11(9):1534. doi: 10.3390/cells11091534. Cells. 2022. PMID: 35563839 Free PMC article. Review.
-
Designing 3-Dimensional In Vitro Oviduct Culture Systems to Study Mammalian Fertilization and Embryo Production.Ann Biomed Eng. 2017 Jul;45(7):1731-1744. doi: 10.1007/s10439-016-1760-x. Epub 2016 Nov 14. Ann Biomed Eng. 2017. PMID: 27844174 Free PMC article. Review.
Cited by
-
Organ-on-a-chip: future of female reproductive pathophysiological models.J Nanobiotechnology. 2024 Jul 31;22(1):455. doi: 10.1186/s12951-024-02651-w. J Nanobiotechnology. 2024. PMID: 39085921 Free PMC article. Review.
-
Revolutionizing the female reproductive system research using microfluidic chip platform.J Nanobiotechnology. 2023 Dec 19;21(1):490. doi: 10.1186/s12951-023-02258-7. J Nanobiotechnology. 2023. PMID: 38111049 Free PMC article. Review.
-
Oxygen levels affect oviduct epithelium functions in air-liquid interface culture.Histochem Cell Biol. 2024 Jun;161(6):521-537. doi: 10.1007/s00418-024-02273-1. Epub 2024 Mar 26. Histochem Cell Biol. 2024. PMID: 38530407 Free PMC article.
-
Induction of in vivo-like ciliation in confluent monolayers of re-differentiated equine oviduct epithelial cells†.Biol Reprod. 2024 Sep 14;111(3):580-599. doi: 10.1093/biolre/ioae090. Biol Reprod. 2024. PMID: 38847468 Free PMC article.
-
Microfluidic chips in female reproduction: a systematic review of status, advances, and challenges.Theranostics. 2024 Jul 15;14(11):4352-4374. doi: 10.7150/thno.97301. eCollection 2024. Theranostics. 2024. PMID: 39113805 Free PMC article.
References
-
- Palmer E, Bezard J, Magistrini M, Duchamp G. In vitro fertilization in the horse. A retrospective study. J Reprod Fertil Supp 1991; 44:375–384. - PubMed
-
- Bézard J, Magistrini M, Battut I, Duchamp G, Palmer E. In vitro fertilization in the mare. Recueil De Medecine Veterinaire 1992; 168:993–1003.
-
- Hinrichs K, Love CC, Brinsko SP, Choi YH, Varner DD. In vitro fertilization of in vitro-matured equine oocytes: effect of maturation medium, duration of maturation, and sperm calcium ionophore treatment, and comparison with rates of fertilization in vivo after oviductal transfer. Biol Reprod 2002; 67:256–262. - PubMed
-
- Choi YH, Okada Y, Hochi S, Braun J, Sato K, Oguri N. In-vitro fertilization rate of horse oocytes with partially removed zonae. Theriogenology 1994; 42:795–802. - PubMed
-
- McPartlin LA, Suarez SS, Czaya CA, Hinrichs K, Bedford-Guaus SJ. Hyperactivation of stallion sperm is required for successful in vitro fertilization of equine oocytes. Biol Reprod 2009; 81:199–206. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Research Materials

