Long-term Effectiveness of Adjuvant Treatment With Catechol-O-Methyltransferase or Monoamine Oxidase B Inhibitors Compared With Dopamine Agonists Among Patients With Parkinson Disease Uncontrolled by Levodopa Therapy: The PD MED Randomized Clinical Trial
- PMID: 34962574
- PMCID: PMC8715387
- DOI: 10.1001/jamaneurol.2021.4736
Long-term Effectiveness of Adjuvant Treatment With Catechol-O-Methyltransferase or Monoamine Oxidase B Inhibitors Compared With Dopamine Agonists Among Patients With Parkinson Disease Uncontrolled by Levodopa Therapy: The PD MED Randomized Clinical Trial
Abstract
Importance: Many people with Parkinson disease (PD) develop motor complications that are uncontrolled by levodopa dose adjustment. Among these patients, it is uncertain which drug class is more effective as adjuvant therapy.
Objective: To compare the long-term effects on patient-rated quality of life of adding a dopamine agonist vs a dopamine reuptake inhibitor (DRI), either a monoamine oxidase type B (MAO-B) inhibitor or a catechol-O-methyltransferase (COMT) inhibitor, to levodopa therapy for the treatment of patients with motor complications of PD.
Design, setting, and participants: This pragmatic semifactorial (2 × 1) randomized clinical trial recruited from 64 neurology and geriatric clinics (62 in the United Kingdom, 1 in the Czech Republic, and 1 in Russia) between February 23, 2001, and December 15, 2009. A total of 500 patients with idiopathic PD who developed uncontrolled motor complications and did not have dementia were randomly assigned on a 1:1:1 basis using a computerized minimization program. Data were analyzed between 2017 and 2020.
Interventions: Open-label dopamine agonist, MAO-B inhibitor, or COMT inhibitor.
Main outcomes and measures: Primary outcomes were scores on the 39-item Parkinson's Disease Questionnaire (PDQ-39) mobility domain and cost-effectiveness. Outcomes were assessed before study entry, at 6 and 12 months after randomization, and annually thereafter. Repeated-measures and log rank analyses were used in an intention-to-treat approach.
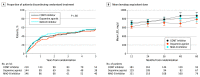
Results: Among 500 participants, the mean (SD) age was 73.0 (8.2) years; 314 participants (62.8%) were men. Over a median of 4.5 years (range, 0-13.3 years) of follow-up, the participants in the dopamine agonist group had a mean PDQ-39 mobility score that was 2.4 points (95% CI, -1.3 to 6.0 points) better than that of the combined MAO-B and COMT groups; however, this difference was not significant (P=.20). With regard to DRIs, participants in the MAO-B group had mean PDQ-39 mobility scores that were 4.2 points (95% CI, 0.4-7.9 points; P=.03) better than those of the COMT group and EuroQol 5-dimension 3-level (EQ-5D-3L) utility scores that were 0.05 points (95% CI, 0.003-0.09 points; P=.04) better than the COMT group. Nonsignificant improvements were found in the PDQ-39 summary index (mean difference, 2.2 points; 95% CI, -0.2 to 4.5 points; P=.07) along with nonsignificant reductions in dementia (rate ratio [RR], 0.70; 95% CI, 0.47-1.03; P = .07) and mortality (RR, 0.76; 95% CI, 0.56-1.03; P=.07). When dopamine agonists were compared with MAO-B inhibitors only, the outcomes were similar.
Conclusions and relevance: In this study, patient-rated quality of life was inferior when COMT inhibitors were used as adjuvant treatment compared with MAO-B inhibitors or dopamine agonists among people with PD who experienced motor complications that were uncontrolled by levodopa therapy. The MAO-B inhibitors produced equivalent disease control, suggesting that these agents may be underused as adjuvant therapy.
Trial registration: isrctn.org Identifier: ISRCTN69812316; EU Clinical Trials Register Identifier: 2005-001813-16.
Conflict of interest statement
Figures


Comment in
-
Adjunctive Therapies in Parkinson Disease-Have We Made Meaningful Progress?JAMA Neurol. 2022 Feb 1;79(2):119-120. doi: 10.1001/jamaneurol.2021.4140. JAMA Neurol. 2022. PMID: 34962569 No abstract available.
-
Commentary on Long-term Effectiveness of Adjuvant Treatment in Parkinson Disease-Reply.JAMA Neurol. 2022 Jul 1;79(7):726-727. doi: 10.1001/jamaneurol.2022.1604. JAMA Neurol. 2022. PMID: 35759259 No abstract available.
-
Commentary on Long-term Effectiveness of Adjuvant Treatment in Parkinson Disease.JAMA Neurol. 2022 Jul 1;79(7):726. doi: 10.1001/jamaneurol.2022.1601. JAMA Neurol. 2022. PMID: 35759267 No abstract available.
References
-
- Gray R, Ives N, Rick C, et al. ; PD MED Collaborative Group . Long-term effectiveness of dopamine agonists and monoamine oxidase B inhibitors compared with levodopa as initial treatment for Parkinson’s disease (PD MED): a large, open-label, pragmatic randomised trial. Lancet. 2014;384(9949):1196-1205. doi: 10.1016/S0140-6736(14)60683-8 - DOI - PubMed
-
- National Institute for Health and Care Excellence . Parkinson’s Disease in Adults: Diagnosis and Management Guideline. National Institute for Health and Care Excellence; 2017. NICE guideline NG71. Accessed August 8, 2017. https://www.nice.org.uk/guidance/ng71/evidence/full-guideline-pdf-453846...
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

