Correlations Between Root Metabolomics and Bacterial Community Structures in the Phragmites australis Under Acid Mine Drainage-Polluted Wetland Ecosystem
- PMID: 34962589
- PMCID: PMC8714630
- DOI: 10.1007/s00284-021-02748-7
Correlations Between Root Metabolomics and Bacterial Community Structures in the Phragmites australis Under Acid Mine Drainage-Polluted Wetland Ecosystem
Abstract
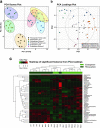
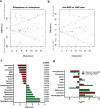
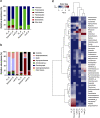
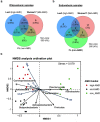
Despite root microecology playing critical role in plant growth and fidelity, relatively few studies have focused on the link between the microbial communities and root metabolome in the aquatic macrophytes under heavy metal (HM) pollution. Using high-throughput metagenomic sequencing, targeted metabolomics and community-level physiological profile analyses, we investigated the symbiotic associations between Phragmites australis with rhizospheric bacterial communities under differing acid mine drainage (AMD) pollution. Results indicated that AMD pollution and root localization significantly affected root metabolome profiles. Higher accumulation of adenosine monophosphate, inosine, methionine, carnitine and dimethylglycine were observed in the rhizosphere under AMD than non-AMD habitat. Overall, the bacterial diversity and richness, and functional (metabolic) diversity were lower under high-AMD pollution. While non-AMD site was enriched with members of phylum Firmicutes, Proteobacteria were the most abundant taxa in the rhizosphere and endosphere under AMD-polluted sites. Further, plant growth promoting rhizobacteria (Rhizobium, Delftia, Bradyrhizobium, and Mesorhizobium) and metal-tolerant bacteria (Bacillus, Arthrobacter, Massilia and Methylocystis) were most abundant in AMD-polluted than non-AMD habitat. Finally, pH, TDS (total dissolved solids), Cu, Cr, Fe, and Zn content were the key environmental factors that strongly contributed to the spatial perturbation of rhizospheric metabolites, proteobacterial and acidobacterial taxa. Overall, the study linked the differential endospheric and rhizospheric bacterial community and metabolite profiles in P. australis under AMD environment and provided insights into HM adaptability and phytoremediation potential.
© 2021. The Author(s).
Conflict of interest statement
Chimdi Mang Kalu, Henry Joseph Oduor Ogola, Ramganesh Selvarajan, Memory Tekere and Khayalethu Ntushelo declare no conflict of interest.
Figures
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

