Early manifestation of aging-related vascular dysfunction in human penile vasculature-A potential explanation for the role of erectile dysfunction as a harbinger of systemic vascular disease
- PMID: 34962617
- PMCID: PMC8811115
- DOI: 10.1007/s11357-021-00507-x
Early manifestation of aging-related vascular dysfunction in human penile vasculature-A potential explanation for the role of erectile dysfunction as a harbinger of systemic vascular disease
Abstract
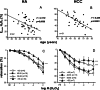
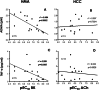
Advanced age is related to functional alterations of human vasculature, but erectile dysfunction precedes systemic manifestations of vascular disease. The current study aimed to simultaneously evaluate the influence of aging on vascular function (relaxation and contraction responses) in systemic human vascular territories: aorta (HA) and resistance mesenteric arteries (HMA) and human corpus cavernosum (HCC) and penile resistance arteries (HPRA). Associations of oxidative stress and inflammation circulating biomarkers with age and functional responses were also determined. Vascular specimens were obtained from 76 organ donors (age range 18-87). Four age-groups were established: < 40, 40-55, 56-65 and > 65 years old. Increasing age was associated with a decline in endothelium-dependent relaxation induced by BK in HMA (r = -0.597, p = 0.0001), or by ACh in HCC (r = -0.505, p = 0.0022), and HPRA (r = -0.601, p = 0.0012). Significant impairment was detected at > 65 years old in HMA but earlier in penile vasculature (> 55 years old). Age-related reduction to H2O2-vasodilatory response started before in HCC (56-65 years old) than in HA (> 65 years old). In contrast to relaxation responses, aging-related hypercontractility to adrenergic stimulation was homogeneous: contractions significantly increased in subjects > 55 years old in all tested vessels. Although not significantly age related, circulating levels of ADMA (r = -0.681, p = 0.0052) and TNF-α (r = -0.537, p = 0.0385) were negatively correlated with endothelial vasodilation in HMA but not in HCC or HPRA. Penile vasculature exhibits an early impairment of endothelium-dependent and H2O2-induced vasodilations when compared to mesenteric microcirculation and aorta. Therefore, functional susceptibility of penile vasculature to the aging process may account for anticipation of erectile dysfunction to systemic manifestations of vascular disease.
Keywords: Aorta; Biomarkers; Corpus cavernosum; Endothelial vasodilation; Erectile dysfunction; Human; Microvasculature; Vascular aging.
© 2021. The Author(s), under exclusive licence to American Aging Association.
Conflict of interest statement
Authors declare that they have no conflicts of interest to disclose.
Figures


Similar articles
-
Nitrergic function is lost but endothelial function is preserved in the corpus cavernosum and penile resistance arteries of men after radical prostatectomy.J Sex Med. 2015 Mar;12(3):590-9. doi: 10.1111/jsm.12801. Epub 2014 Dec 21. J Sex Med. 2015. PMID: 25529966
-
Short-term pharmacological activation of Nrf2 ameliorates vascular dysfunction in aged rats and in pathological human vasculature. A potential target for therapeutic intervention.Redox Biol. 2019 Sep;26:101271. doi: 10.1016/j.redox.2019.101271. Epub 2019 Jul 5. Redox Biol. 2019. PMID: 31302408 Free PMC article.
-
Erectile dysfunction is associated with defective L-cysteine/hydrogen sulfide pathway in human corpus cavernosum and penile arteries.Eur J Pharmacol. 2020 Oct 5;884:173370. doi: 10.1016/j.ejphar.2020.173370. Epub 2020 Jul 23. Eur J Pharmacol. 2020. PMID: 32712093
-
Penile arteries and erection.J Vasc Res. 2002 Jul-Aug;39(4):283-303. doi: 10.1159/000065541. J Vasc Res. 2002. PMID: 12187119 Review.
-
Physiological regulation of penile arteries and veins.Int J Impot Res. 2008 Jan-Feb;20(1):17-29. doi: 10.1038/sj.ijir.3901581. Epub 2007 Jul 19. Int J Impot Res. 2008. PMID: 17637789 Review.
Cited by
-
Targeting TRPC-5 Channel Inhibition to Improve Penile Vascular Function in Erectile Dysfunction.Int J Mol Sci. 2025 Feb 8;26(4):1431. doi: 10.3390/ijms26041431. Int J Mol Sci. 2025. PMID: 40003900 Free PMC article.
-
Risk factors for erectile dysfunction in diabetes mellitus: a systematic review and meta-analysis.Front Endocrinol (Lausanne). 2024 Apr 4;15:1368079. doi: 10.3389/fendo.2024.1368079. eCollection 2024. Front Endocrinol (Lausanne). 2024. PMID: 38638136 Free PMC article.
-
Long-term administration of resveratrol and MitoQ stimulates cavernosum antioxidant gene expression in a mouse castration model of erectile dysfunction.Life Sci. 2022 Dec 1;310:121082. doi: 10.1016/j.lfs.2022.121082. Epub 2022 Oct 15. Life Sci. 2022. PMID: 36252696 Free PMC article.
-
Functional Role of STIM-1 and Orai1 in Human Microvascular Aging.Cells. 2022 Nov 18;11(22):3675. doi: 10.3390/cells11223675. Cells. 2022. PMID: 36429103 Free PMC article.
-
Upregulation of Orai Channels Contributes to Aging-Related Vascular Alterations in Rat Coronary Arteries.Int J Mol Sci. 2023 Aug 29;24(17):13402. doi: 10.3390/ijms241713402. Int J Mol Sci. 2023. PMID: 37686206 Free PMC article.
References
-
- United Nations. World Population Ageing 2020. Highlights. https://www.un.org/development/desa/pd/sites/www.un.org.development.desa....
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

