Lack of p62 Impairs Glycogen Aggregation and Exacerbates Pathology in a Mouse Model of Myoclonic Epilepsy of Lafora
- PMID: 34962634
- PMCID: PMC8857170
- DOI: 10.1007/s12035-021-02682-6
Lack of p62 Impairs Glycogen Aggregation and Exacerbates Pathology in a Mouse Model of Myoclonic Epilepsy of Lafora
Abstract
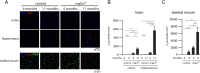
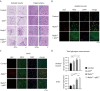
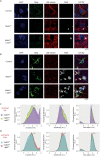
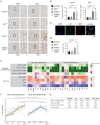
Lafora disease (LD) is a fatal childhood-onset dementia characterized by the extensive accumulation of glycogen aggregates-the so-called Lafora Bodies (LBs)-in several organs. The accumulation of LBs in the brain underlies the neurological phenotype of the disease. LBs are composed of abnormal glycogen and various associated proteins, including p62, an autophagy adaptor that participates in the aggregation and clearance of misfolded proteins. To study the role of p62 in the formation of LBs and its participation in the pathology of LD, we generated a mouse model of the disease (malinKO) lacking p62. Deletion of p62 prevented LB accumulation in skeletal muscle and cardiac tissue. In the brain, the absence of p62 altered LB morphology and increased susceptibility to epilepsy. These results demonstrate that p62 participates in the formation of LBs and suggest that the sequestration of abnormal glycogen into LBs is a protective mechanism through which it reduces the deleterious consequences of its accumulation in the brain.
Keywords: Epilepsy; Glycogen; Lafora bodies; Lafora disease; Malin; Neuroinflammation; p62.
© 2021. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


Similar articles
-
Lithium exacerbates Lafora body formation in the Epm2a-/- Lafora disease mouse model.Neurosci Lett. 2025 May 29;856-858:138250. doi: 10.1016/j.neulet.2025.138250. Epub 2025 Apr 19. Neurosci Lett. 2025. PMID: 40258565
-
An inducible glycogen synthase-1 knockout halts but does not reverse Lafora disease progression in mice.J Biol Chem. 2021 Jan-Jun;296:100150. doi: 10.1074/jbc.RA120.015773. Epub 2020 Dec 10. J Biol Chem. 2021. PMID: 33277363 Free PMC article.
-
Glycogen accumulation underlies neurodegeneration and autophagy impairment in Lafora disease.Hum Mol Genet. 2014 Jun 15;23(12):3147-56. doi: 10.1093/hmg/ddu024. Epub 2014 Jan 22. Hum Mol Genet. 2014. PMID: 24452334
-
Therapeutic Window for the Treatment of Lafora Disease.In: Noebels JL, Avoli M, Rogawski MA, Vezzani A, Delgado-Escueta AV, editors. Jasper's Basic Mechanisms of the Epilepsies. 5th edition. New York: Oxford University Press; 2024. Chapter 53. In: Noebels JL, Avoli M, Rogawski MA, Vezzani A, Delgado-Escueta AV, editors. Jasper's Basic Mechanisms of the Epilepsies. 5th edition. New York: Oxford University Press; 2024. Chapter 53. PMID: 39637182 Free Books & Documents. Review.
-
Treating Lafora Disease with an Antibody-Enzyme Fusion.In: Noebels JL, Avoli M, Rogawski MA, Vezzani A, Delgado-Escueta AV, editors. Jasper's Basic Mechanisms of the Epilepsies. 5th edition. New York: Oxford University Press; 2024. Chapter 55. In: Noebels JL, Avoli M, Rogawski MA, Vezzani A, Delgado-Escueta AV, editors. Jasper's Basic Mechanisms of the Epilepsies. 5th edition. New York: Oxford University Press; 2024. Chapter 55. PMID: 39637175 Free Books & Documents. Review.
Cited by
-
Role of Astrocytes in the Pathophysiology of Lafora Disease and Other Glycogen Storage Disorders.Cells. 2023 Feb 24;12(5):722. doi: 10.3390/cells12050722. Cells. 2023. PMID: 36899857 Free PMC article. Review.
-
Autophagy-related genes in mesial temporal lobe epilepsy: an integrated bioinformatics analysis.Acta Epileptol. 2024 Apr 25;6(1):16. doi: 10.1186/s42494-024-00160-9. Acta Epileptol. 2024. PMID: 40217519 Free PMC article.
-
Glial Contributions to Lafora Disease: A Systematic Review.Biomedicines. 2022 Dec 1;10(12):3103. doi: 10.3390/biomedicines10123103. Biomedicines. 2022. PMID: 36551859 Free PMC article. Review.
-
Malin restoration as proof of concept for gene therapy for Lafora disease.Brain Commun. 2022 Jun 23;4(4):fcac168. doi: 10.1093/braincomms/fcac168. eCollection 2022. Brain Commun. 2022. PMID: 35813879 Free PMC article.
-
Brain Glycogen-Its Metabolic Role in Neuronal Health and Neurological Disorders-An Extensive Narrative Review.Metabolites. 2025 Feb 13;15(2):128. doi: 10.3390/metabo15020128. Metabolites. 2025. PMID: 39997753 Free PMC article. Review.
References
-
- Cataldo AM, Broadwell RD. Cytochemical identification of cerebral glycogen and glucose-6-phosphatase activity under normal and experimental conditions. II. Choroid plexus and ependymal epithelia, endothelia and pericytes. J Neurocytol. 1986;15(4):511–24. - PubMed
-
- Duran J, Guinovart JJ. Brain glycogen in health and disease. Mol Aspects Med. 2015;46:70–77. - PubMed
-
- Saez I, Duran J, Sinadinos C, Beltran A, Yanes O, Tevy MF, et al. Neurons have an active glycogen metabolism that contributes to tolerance to hypoxia. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 2014;34(6):945–955. - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

