Combination treatment optimization using a pan-cancer pathway model
- PMID: 34962919
- PMCID: PMC8747684
- DOI: 10.1371/journal.pcbi.1009689
Combination treatment optimization using a pan-cancer pathway model
Abstract
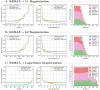
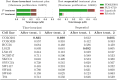
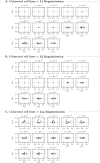
The design of efficient combination therapies is a difficult key challenge in the treatment of complex diseases such as cancers. The large heterogeneity of cancers and the large number of available drugs renders exhaustive in vivo or even in vitro investigation of possible treatments impractical. In recent years, sophisticated mechanistic, ordinary differential equation-based pathways models that can predict treatment responses at a molecular level have been developed. However, surprisingly little effort has been put into leveraging these models to find novel therapies. In this paper we use for the first time, to our knowledge, a large-scale state-of-the-art pan-cancer signaling pathway model to identify candidates for novel combination therapies to treat individual cancer cell lines from various tissues (e.g., minimizing proliferation while keeping dosage low to avoid adverse side effects) and populations of heterogeneous cancer cell lines (e.g., minimizing the maximum or average proliferation across the cell lines while keeping dosage low). We also show how our method can be used to optimize the drug combinations used in sequential treatment plans-that is, optimized sequences of potentially different drug combinations-providing additional benefits. In order to solve the treatment optimization problems, we combine the Covariance Matrix Adaptation Evolution Strategy (CMA-ES) algorithm with a significantly more scalable sampling scheme for truncated Gaussian distributions, based on a Hamiltonian Monte-Carlo method. These optimization techniques are independent of the signaling pathway model, and can thus be adapted to find treatment candidates for other complex diseases than cancers as well, as long as a suitable predictive model is available.
Conflict of interest statement
I have read the journal’s policy and the authors of this manuscript have the following competing interests: TS is Founder, President, and CEO of Sandholm Enterprises, Ltd., Strategic Machine, Inc., Strategy Robot, Inc., and Optimized Markets, Inc.; these affiliations did not affect the conclusions of this paper.
Figures
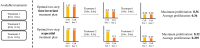


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

